当前位置:
X-MOL 学术
›
Chemosphere
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of N-(2,6-dimethylphenyl)hydroxylamine adducts of 2'-deoxyguanosine under weakly basic conditions.
Chemosphere ( IF 8.1 ) Pub Date : 2020-03-20 , DOI: 10.1016/j.chemosphere.2020.126530 Takuya Matsui 1 , Naohito Yamada 2 , Hideyuki Kuno 2 , Robert A Kanaly 3
Chemosphere ( IF 8.1 ) Pub Date : 2020-03-20 , DOI: 10.1016/j.chemosphere.2020.126530 Takuya Matsui 1 , Naohito Yamada 2 , Hideyuki Kuno 2 , Robert A Kanaly 3
Affiliation
|
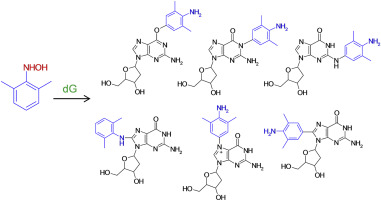
Aromatic amines are a class of chemical carcinogens that are activated by cytochrome P450 enzymes to form arylhydroxylamines that are conjugated to form N-acetoxyarylamines or N-sulfonyloxyarylamines. These conjugates undergo N-O bond cleavage to become reactive nitrenium ions that may form DNA adducts. Numerous studies in the past using N-acetoxyarylamines to investigate DNA adduct formation were conducted, however, less is known in regard to DNA adduct formation directly from arylhydroxylamines - especially under conditions that mimic the physiological conditions of cells such as weakly basic conditions. In this study, 2'-deoxyguanosine (dG) was exposed to N-(2,6-dimethylphenyl)hydroxylamine (2,6-DMPHA) and N-phenylhydroxylamine (PHA) at pH 7.4 without enzymes and analyzed by liquid chromatography high resolution mass spectrometry (LC-HRMS). 2,6-DMPHA exposure resulted in the production of relatively low amounts of adducts however the identities of at least six different adducts that were formed through reactions with carbon, nitrogen and oxygen of 2'-deoxyguanosine were proposed based upon different analytical approaches including HRMS CID fragmentation and NMR analyses. Contrastively, PHA exposure under identical conditions resulted in one adduct at the C8 position. It was concluded from these results and results of theoretical calculations that nitrenium ions produced from 2,6-DMPHA were relatively more stable resulting in longer nitrenium ion lifetimes which ultimately led to greater potential for 2,6-DMPHA nitrenium ions to react with multiple sites on dG.
中文翻译:

弱碱性条件下2'-脱氧鸟苷的N-(2,6-二甲基苯基)羟胺加合物的表征。
芳香胺是一类化学致癌物,可被细胞色素P450酶活化以形成芳基羟胺,这些芳基羟胺经共轭可形成N-乙酰氧基芳基胺或N-磺酰氧基芳基胺。这些共轭物经历NO键断裂,成为可形成DNA加合物的反应性氮离子。过去使用N-乙酰氧基芳基胺来研究DNA加合物的形成已经进行了许多研究,但是,关于直接由芳基羟胺形成DNA加合物的了解较少,特别是在模仿细胞生理条件的条件下,例如弱碱性条件。在这项研究中,将2'-脱氧鸟苷(dG)暴露于pH 7.4的N-(2,6-二甲基苯基)羟胺(2,6-DMPHA)和N-苯基羟胺(PHA),并通过液相色谱高分辨率分析质谱(LC-HRMS)。暴露于2,6-DMPHA导致生成的加合物数量相对较低,但是基于包括HRMS在内的不同分析方法,提出了至少2种与2'-脱氧鸟苷的碳,氮和氧反应形成的不同加合物的身份CID片段化和NMR分析。相反,在相同条件下暴露PHA会在C8位置产生一个加合物。从这些结果和理论计算结果可以得出结论,由2,6-DMPHA产生的亚硝酸根离子相对更稳定,导致更长的亚硝酸根离子寿命,最终导致2,6-DMPHA亚硝酸根离子具有与多个位点反应的更大潜力在dG上。6-DMPHA暴露导致生成的加合物数量相对较低,但是基于包括HRMS CID裂解在内的不同分析方法,提出了至少2种通过与2'-脱氧鸟苷的碳,氮和氧反应形成的不同加合物的身份和NMR分析。相反,在相同条件下暴露PHA会在C8位置产生一个加合物。从这些结果和理论计算结果可以得出结论,由2,6-DMPHA产生的亚硝酸根离子相对更稳定,导致更长的亚硝酸根离子寿命,最终导致2,6-DMPHA亚硝酸根离子具有与多个位点反应的更大潜力在dG上。6-DMPHA暴露导致生成的加合物数量相对较低,但是基于包括HRMS CID裂解在内的不同分析方法,提出了至少2种通过与2'-脱氧鸟苷的碳,氮和氧反应形成的不同加合物的身份和NMR分析。相反,在相同条件下暴露PHA会在C8位置产生一个加合物。从这些结果和理论计算结果可以得出结论,由2,6-DMPHA产生的亚硝酸根离子相对更稳定,导致更长的亚硝酸根离子寿命,最终导致2,6-DMPHA亚硝酸根离子具有与多个位点反应的更大潜力在dG上。基于不同的分析方法,包括HRMS CID片段化和NMR分析,提出了脱氧鸟苷。相反,在相同条件下暴露PHA会在C8位置产生一个加合物。从这些结果和理论计算结果可以得出结论,由2,6-DMPHA产生的亚硝酸根离子相对更稳定,导致更长的亚硝酸根离子寿命,最终导致2,6-DMPHA亚硝酸根离子具有与多个位点反应的更大潜力在dG上。基于不同的分析方法,包括HRMS CID片段化和NMR分析,提出了脱氧鸟苷。相反,在相同条件下暴露PHA会在C8位置产生一个加合物。从这些结果和理论计算结果可以得出结论,由2,6-DMPHA产生的亚硝酸根离子相对更稳定,从而导致更长的亚硝酸根离子寿命,最终导致2,6-DMPHA亚硝酸根离子具有与多个位点反应的更大潜力。在dG上。
更新日期:2020-03-21
中文翻译:

弱碱性条件下2'-脱氧鸟苷的N-(2,6-二甲基苯基)羟胺加合物的表征。
芳香胺是一类化学致癌物,可被细胞色素P450酶活化以形成芳基羟胺,这些芳基羟胺经共轭可形成N-乙酰氧基芳基胺或N-磺酰氧基芳基胺。这些共轭物经历NO键断裂,成为可形成DNA加合物的反应性氮离子。过去使用N-乙酰氧基芳基胺来研究DNA加合物的形成已经进行了许多研究,但是,关于直接由芳基羟胺形成DNA加合物的了解较少,特别是在模仿细胞生理条件的条件下,例如弱碱性条件。在这项研究中,将2'-脱氧鸟苷(dG)暴露于pH 7.4的N-(2,6-二甲基苯基)羟胺(2,6-DMPHA)和N-苯基羟胺(PHA),并通过液相色谱高分辨率分析质谱(LC-HRMS)。暴露于2,6-DMPHA导致生成的加合物数量相对较低,但是基于包括HRMS在内的不同分析方法,提出了至少2种与2'-脱氧鸟苷的碳,氮和氧反应形成的不同加合物的身份CID片段化和NMR分析。相反,在相同条件下暴露PHA会在C8位置产生一个加合物。从这些结果和理论计算结果可以得出结论,由2,6-DMPHA产生的亚硝酸根离子相对更稳定,导致更长的亚硝酸根离子寿命,最终导致2,6-DMPHA亚硝酸根离子具有与多个位点反应的更大潜力在dG上。6-DMPHA暴露导致生成的加合物数量相对较低,但是基于包括HRMS CID裂解在内的不同分析方法,提出了至少2种通过与2'-脱氧鸟苷的碳,氮和氧反应形成的不同加合物的身份和NMR分析。相反,在相同条件下暴露PHA会在C8位置产生一个加合物。从这些结果和理论计算结果可以得出结论,由2,6-DMPHA产生的亚硝酸根离子相对更稳定,导致更长的亚硝酸根离子寿命,最终导致2,6-DMPHA亚硝酸根离子具有与多个位点反应的更大潜力在dG上。6-DMPHA暴露导致生成的加合物数量相对较低,但是基于包括HRMS CID裂解在内的不同分析方法,提出了至少2种通过与2'-脱氧鸟苷的碳,氮和氧反应形成的不同加合物的身份和NMR分析。相反,在相同条件下暴露PHA会在C8位置产生一个加合物。从这些结果和理论计算结果可以得出结论,由2,6-DMPHA产生的亚硝酸根离子相对更稳定,导致更长的亚硝酸根离子寿命,最终导致2,6-DMPHA亚硝酸根离子具有与多个位点反应的更大潜力在dG上。基于不同的分析方法,包括HRMS CID片段化和NMR分析,提出了脱氧鸟苷。相反,在相同条件下暴露PHA会在C8位置产生一个加合物。从这些结果和理论计算结果可以得出结论,由2,6-DMPHA产生的亚硝酸根离子相对更稳定,导致更长的亚硝酸根离子寿命,最终导致2,6-DMPHA亚硝酸根离子具有与多个位点反应的更大潜力在dG上。基于不同的分析方法,包括HRMS CID片段化和NMR分析,提出了脱氧鸟苷。相反,在相同条件下暴露PHA会在C8位置产生一个加合物。从这些结果和理论计算结果可以得出结论,由2,6-DMPHA产生的亚硝酸根离子相对更稳定,从而导致更长的亚硝酸根离子寿命,最终导致2,6-DMPHA亚硝酸根离子具有与多个位点反应的更大潜力。在dG上。

































 京公网安备 11010802027423号
京公网安备 11010802027423号