当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
What Is the Transfer Mechanism of Photoexcited Charge Carriers for g-C3N4/TiO2 Heterojunction Photocatalysts? Verification of the Relative p–n Junction Theory
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.jpcc.0c00422 Nannan Yuan 1 , Jinfeng Zhang 1 , Sujuan Zhang 1 , Gaoli Chen 1 , Sugang Meng 1 , You Fan 1 , Xiuzhen Zheng 1 , Shifu Chen 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.jpcc.0c00422 Nannan Yuan 1 , Jinfeng Zhang 1 , Sujuan Zhang 1 , Gaoli Chen 1 , Sugang Meng 1 , You Fan 1 , Xiuzhen Zheng 1 , Shifu Chen 1
Affiliation
|
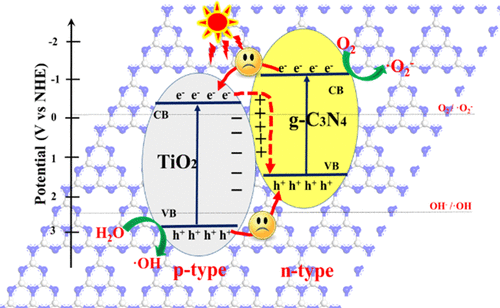
Investigation of photoexcited charge transfer mechanism has always been one of the hotspots of the photocatalysis field. In our recent studies, the relative p–n junction was proposed as a new concept, and the built-in electric field formed in the heterojunction is the inner impetus for driving photoexcited charge transfer. Here, a series of g-C3N4/TiO2 samples with different mass percentage contents were synthesized and further characterized by physical and chemical techniques for the investigation of the charge transfer mechanism and internal natural law. The results state clearly that the migration of photoexcited charges belongs to Z-scheme mechanism, which is suitable for as-synthesized g-C3N4/TiO2 samples, whether the main part of the g-C3N4/TiO2 is TiO2 or g-C3N4. The photoexcited electrons enriched in g-C3N4 with a higher negative conduction band (CB) potential have reduction ability to convert O2 into superoxide radicals (•O2–). Meanwhile, the photoexcited holes in TiO2 with a higher positive valence band (VB) potential have oxidation ability to activate H2O or hydroxyl ions (OH–) to hydroxyl radicals (•OH). Furthermore, the g-C3N4/TiO2 photocatalyst exhibits better photocatalytic performance than TiO2 and g-C3N4. It is encouraging that the abovementioned Z-scheme mechanism of photoexcited charge transfer can also be explained and confirmed by the relative p–n junction theory. The built-in electric field promotes the migration of the photoexcited charges in the heterojunction, and its migration direction is opposite to that of the photoexcited charge in the CB and VB of g-C3N4 and TiO2. Therefore, the relative p–n junction theory not only is used to explicate the migration mechanism and internal natural law of the photoexcited charge in the heterojunction photocatalysts but also has crucial guiding significance for the theoretical design and practical construction of composite photocatalysts.
中文翻译:

gC 3 N 4 / TiO 2异质结光催化剂的光激发电荷载体的传递机理是什么?相对的验证p - ñ结理论
光激发电荷转移机理的研究一直是光催化领域的热点之一。在我们最近的研究中,提出了相对p - n结作为一个新概念,异质结中形成的内置电场是驱动光激发电荷转移的内在动力。在此,合成了一系列具有不同质量百分比含量的gC 3 N 4 / TiO 2样品,并通过物理和化学技术对其进行了表征,以研究电荷转移机理和内部自然规律。结果清楚地表明,光激发电荷的迁移属于Z-scheme机制,这是适合于合成后原样的GC 3 Ñ 4 /二氧化钛2个样品的GC主要部分是否3 Ñ 4 /二氧化钛2为TiO 2或GC 3 ñ 4。在GC富集的光激发电子3 Ñ 4具有更高的负导带(CB)潜在具有将O简化能力2到超氧自由基(•Ø 2 - )。同时,具有较高正价带(VB)势的TiO 2中的光激发空穴具有氧化能力以激活H 2。O或氢氧根离子(OH –)形成羟基(•OH)。此外,gC 3 N 4 / TiO 2光催化剂表现出比TiO 2和gC 3 N 4更好的光催化性能。令人鼓舞的是,上述Z型光激发电荷转移的机理也可以通过相对p - n结理论加以解释和证实。内置电场促进了异质结中光激发电荷的迁移,其迁移方向与gC 3 N 4的CB和VB中的光激发电荷的迁移方向相反。和TiO 2。因此,相对p - ñ结理论不仅被用来阐明的迁移机制,并在异质结光催化剂的光激发电荷的内部自然法则,但也有复合光催化剂的理论设计和实际建设至关重要的指导意义。
更新日期:2020-04-24
中文翻译:

gC 3 N 4 / TiO 2异质结光催化剂的光激发电荷载体的传递机理是什么?相对的验证p - ñ结理论
光激发电荷转移机理的研究一直是光催化领域的热点之一。在我们最近的研究中,提出了相对p - n结作为一个新概念,异质结中形成的内置电场是驱动光激发电荷转移的内在动力。在此,合成了一系列具有不同质量百分比含量的gC 3 N 4 / TiO 2样品,并通过物理和化学技术对其进行了表征,以研究电荷转移机理和内部自然规律。结果清楚地表明,光激发电荷的迁移属于Z-scheme机制,这是适合于合成后原样的GC 3 Ñ 4 /二氧化钛2个样品的GC主要部分是否3 Ñ 4 /二氧化钛2为TiO 2或GC 3 ñ 4。在GC富集的光激发电子3 Ñ 4具有更高的负导带(CB)潜在具有将O简化能力2到超氧自由基(•Ø 2 - )。同时,具有较高正价带(VB)势的TiO 2中的光激发空穴具有氧化能力以激活H 2。O或氢氧根离子(OH –)形成羟基(•OH)。此外,gC 3 N 4 / TiO 2光催化剂表现出比TiO 2和gC 3 N 4更好的光催化性能。令人鼓舞的是,上述Z型光激发电荷转移的机理也可以通过相对p - n结理论加以解释和证实。内置电场促进了异质结中光激发电荷的迁移,其迁移方向与gC 3 N 4的CB和VB中的光激发电荷的迁移方向相反。和TiO 2。因此,相对p - ñ结理论不仅被用来阐明的迁移机制,并在异质结光催化剂的光激发电荷的内部自然法则,但也有复合光催化剂的理论设计和实际建设至关重要的指导意义。












































 京公网安备 11010802027423号
京公网安备 11010802027423号