当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Mechanisms of Chromium(III) Immobilization by Organo-Ferrihydrite Co-precipitates: The Significant Roles of Ferrihydrite and Carboxyl.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.est.9b06510 Jianjun Yang 1, 2 , Xing Xia 1 , Jin Liu 3 , Jian Wang 4 , Yongfeng Hu 4
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.est.9b06510 Jianjun Yang 1, 2 , Xing Xia 1 , Jin Liu 3 , Jian Wang 4 , Yongfeng Hu 4
Affiliation
|
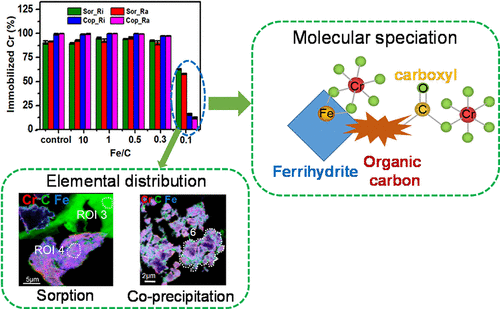
The interaction mechanisms of heavy metals with organo-Fe hydroxides co-precipitates (OFC) remain unclear due to the structural complexity of the OFC. In this study, batch experiments were conducted to investigate the immobilization mechanisms of Cr(III) by the OFC, which was prepared by co-precipitating Fe3+ with rice/rape straw-derived dissolved organic carbon, through sorption and co-precipitation using synchrotron-based X-ray absorption near-edge structure (XANES) spectroscopy and scanning transmission X-ray microscopy (STXM). At an Fe/C molar ratio ≥ 0.3, both the sorption and co-precipitation immobilized the majority of Cr(III), but the co-precipitation desorbed less Cr(III) than the sorption regardless of DOC loadings and sources. In contrast, Cr(III) immobilization was significantly reduced at an Fe/C molar ratio of 0.1 for both reactions. Linear combination fitting of Cr K-edge XANES spectra revealed the predominance of ferrihydrite-bound Cr(III), but enhanced organic Cr(III) occurred with increased organic carbon (OC) loading for both the sorption and co-precipitation. STXM coupled with multi-edge XANES analysis confirmed the primary association of Cr(III) with ferrihydrite and directly probed carboxyl as the binding site for Cr(III) retention on the OC constituents of the OFC. These results provided new molecular-level insights into the Cr(III) retention mechanisms on the OFC, particularly for the interactions of Cr(III) and OC constituents of the OFC, which could benefit the management of Cr-contaminated soils with straw returning.
中文翻译:

有机-铁水合物共沉淀物固定铬(III)的分子机理:铁水合物和羧基的重要作用。
由于OFC的结构复杂性,重金属与有机-Fe-氢氧化物共沉淀物(OFC)的相互作用机理仍不清楚。在这项研究中,我们进行了分批实验,研究了OFC对Cr(III)的固定机理。OFC是通过同步加速器吸附和共沉淀法,将Fe3 +与稻米/强奸稻草衍生的溶解有机碳共沉淀而制备的。基于X射线吸收的近边缘结构(XANES)光谱和扫描透射X射线显微镜(STXM)。在Fe / C摩尔比≥0.3时,吸附和共沉淀都固定了大部分Cr(III),但与DOC负载和来源无关,共沉淀解吸的Cr(III)比吸附少。相反,在两个反应中,Fe / C摩尔比均为0.1时,Cr(III)的固定化明显降低。Cr K边缘XANES光谱的线性组合拟合显示出与水铁矿结合的Cr(III)占优势,但有机Cr(III)的增加伴随着有机碳(OC)的吸附和共沉淀而增加。STXM结合多边缘XANES分析证实了Cr(III)与水铁矿的主要缔合,并直接探测到羧基作为Cr(III)保留在OFC OC成分上的结合位点。这些结果提供了有关分子氧在铬(Ⅲ)上的保留机理的新的分子水平的见解,特别是对于铬(Ⅲ)和氧自由基的OC成分之间的相互作用,这可能有利于秸秆还田对含铬污染土壤的管理。但是在吸附和共沉淀过程中,有机Cr(III)的含量都增加了,而有机碳(OC)的含量也增加了。STXM结合多边缘XANES分析证实了Cr(III)与水铁矿的主要缔合,并直接探测到羧基作为Cr(III)保留在OFC OC成分上的结合位点。这些结果提供了有关分子氧在铬(Ⅲ)上的保留机理的新的分子水平的见解,特别是对于铬(Ⅲ)和氧自由基的OC成分之间的相互作用,这可能有利于秸秆还田对含铬污染土壤的管理。但是在吸附和共沉淀过程中,随着有机碳(OC)含量的增加,有机铬(III)含量增加。STXM结合多边缘XANES分析证实了Cr(III)与水铁矿的主要缔合,并直接探测到羧基作为Cr(III)保留在OFC OC成分上的结合位点。这些结果提供了有关分子氧在铬(Ⅲ)上的保留机理的新的分子水平的见解,特别是对于铬(Ⅲ)和氧自由基的OC成分之间的相互作用,这可能有利于秸秆还田对含铬污染土壤的管理。STXM结合多边缘XANES分析证实了Cr(III)与水铁矿的主要缔合,并直接探测到羧基作为Cr(III)保留在OFC OC成分上的结合位点。这些结果提供了对分子链中的Cr(III)保留机制的新的分子水平的见解,特别是对于OCc中Cr(III)和OC成分之间的相互作用,这可能有利于秸秆还田对含铬污染土壤的管理。STXM结合多边缘XANES分析证实了Cr(III)与水铁矿的主要缔合,并直接探测到羧基作为Cr(III)保留在OFC OC成分上的结合位点。这些结果提供了有关分子氧在铬(Ⅲ)上的保留机理的新的分子水平的见解,特别是对于铬(Ⅲ)和氧自由基的OC成分之间的相互作用,这可能有利于秸秆还田对含铬污染土壤的管理。
更新日期:2020-04-23
中文翻译:

有机-铁水合物共沉淀物固定铬(III)的分子机理:铁水合物和羧基的重要作用。
由于OFC的结构复杂性,重金属与有机-Fe-氢氧化物共沉淀物(OFC)的相互作用机理仍不清楚。在这项研究中,我们进行了分批实验,研究了OFC对Cr(III)的固定机理。OFC是通过同步加速器吸附和共沉淀法,将Fe3 +与稻米/强奸稻草衍生的溶解有机碳共沉淀而制备的。基于X射线吸收的近边缘结构(XANES)光谱和扫描透射X射线显微镜(STXM)。在Fe / C摩尔比≥0.3时,吸附和共沉淀都固定了大部分Cr(III),但与DOC负载和来源无关,共沉淀解吸的Cr(III)比吸附少。相反,在两个反应中,Fe / C摩尔比均为0.1时,Cr(III)的固定化明显降低。Cr K边缘XANES光谱的线性组合拟合显示出与水铁矿结合的Cr(III)占优势,但有机Cr(III)的增加伴随着有机碳(OC)的吸附和共沉淀而增加。STXM结合多边缘XANES分析证实了Cr(III)与水铁矿的主要缔合,并直接探测到羧基作为Cr(III)保留在OFC OC成分上的结合位点。这些结果提供了有关分子氧在铬(Ⅲ)上的保留机理的新的分子水平的见解,特别是对于铬(Ⅲ)和氧自由基的OC成分之间的相互作用,这可能有利于秸秆还田对含铬污染土壤的管理。但是在吸附和共沉淀过程中,有机Cr(III)的含量都增加了,而有机碳(OC)的含量也增加了。STXM结合多边缘XANES分析证实了Cr(III)与水铁矿的主要缔合,并直接探测到羧基作为Cr(III)保留在OFC OC成分上的结合位点。这些结果提供了有关分子氧在铬(Ⅲ)上的保留机理的新的分子水平的见解,特别是对于铬(Ⅲ)和氧自由基的OC成分之间的相互作用,这可能有利于秸秆还田对含铬污染土壤的管理。但是在吸附和共沉淀过程中,随着有机碳(OC)含量的增加,有机铬(III)含量增加。STXM结合多边缘XANES分析证实了Cr(III)与水铁矿的主要缔合,并直接探测到羧基作为Cr(III)保留在OFC OC成分上的结合位点。这些结果提供了有关分子氧在铬(Ⅲ)上的保留机理的新的分子水平的见解,特别是对于铬(Ⅲ)和氧自由基的OC成分之间的相互作用,这可能有利于秸秆还田对含铬污染土壤的管理。STXM结合多边缘XANES分析证实了Cr(III)与水铁矿的主要缔合,并直接探测到羧基作为Cr(III)保留在OFC OC成分上的结合位点。这些结果提供了对分子链中的Cr(III)保留机制的新的分子水平的见解,特别是对于OCc中Cr(III)和OC成分之间的相互作用,这可能有利于秸秆还田对含铬污染土壤的管理。STXM结合多边缘XANES分析证实了Cr(III)与水铁矿的主要缔合,并直接探测到羧基作为Cr(III)保留在OFC OC成分上的结合位点。这些结果提供了有关分子氧在铬(Ⅲ)上的保留机理的新的分子水平的见解,特别是对于铬(Ⅲ)和氧自由基的OC成分之间的相互作用,这可能有利于秸秆还田对含铬污染土壤的管理。

































 京公网安备 11010802027423号
京公网安备 11010802027423号