当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Insight into Palladium(II)–Counterion–Ligand Cooperative Regiodivergent Syntheses of Indolo[3,2-c]coumarins and Benzofuro[3,2-c]quinolinones from Diphenylethyne Derivatives
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-03-18 , DOI: 10.1021/acs.inorgchem.0c00004 Yiying Yang 1 , Yanhong Liu 1 , Rongxiu Zhu 1 , Dongju Zhang 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-03-18 , DOI: 10.1021/acs.inorgchem.0c00004 Yiying Yang 1 , Yanhong Liu 1 , Rongxiu Zhu 1 , Dongju Zhang 1
Affiliation
|
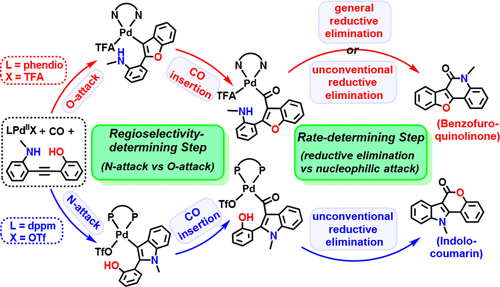
With two distinct active sites, 2-hydroxy-2′-amino-diphenylethyne derivatives can offer benzofuro[3,2-c]quinolinones via the O-attack/N-carbonylation cyclization or indolo[3,2-c]coumarins via the N-attack/O-carbonylation cyclization. This work presents a density functional theory-based computational study to understand the mechanism and origin of the palladium(II)-catalyzed regiodivergent reactivity of diphenylethyne derivatives. It is indicated that the reaction features a palladium(II)–counterion–ligand cooperative catalysis. The O-attack/N-carbonylation cyclization mainly benefits from the inductive effect of the rigid electron-withdrawing bidentate nitrogen ligand and the stabilization of the 3c-4e bond between the trifluoroacetate (TFA) anion and the hydroxyl group in the substrate for the precursor and transition state, while the viability of the N-attack/O-carbonylation cyclization stems intrinsically from the stronger nucleophilicity of the N atom as well as the important π–π interaction between the flexible electron-rich bidentate phosphine ligand and the substrate. Moreover, these calculations propose an unconventional reductive elimination mechanism for the transformation from Pd(II) to Pd(0), where the intramolecular nucleophilic attack of the N/O atom on the carbonyl C atom results in the formal reductive elimination product. The calculated overall barriers of 14.8 kcal/mol for Pd(TFA)2 with the bidentate nitrogen ligand and 23.9 kcal/mol for Pd(OTf)2 with the bidentate phosphine ligand are qualitatively consistent with the mild experimental conditions.
中文翻译:

二苯乙炔衍生物对吲哚[3,2- c ]香豆素和苯并呋喃[3,2- c ]喹啉酮的钯(II)-配体-配体协同区域发散理论研究
2-羟基-2'-氨基-二苯基乙炔衍生物具有两个不同的活性位点,可通过O-攻击/ N-羰基化环化反应生成苯并呋喃[3,2- c ]喹啉酮,或通过C-羟基提供吲哚并[3,2- c ]香豆素。 N攻击/O-羰基化环化。这项工作提出了基于密度泛函理论的计算研究,以了解钯(II)催化的二苯乙炔衍生物的区域发散反应性的机理和起源。结果表明,该反应具有钯(II)-抗衡离子-配体协同催化作用。O-攻击/ N-羰基化环化主要得益于刚性吸电子双齿氮配体的感应作用以及三氟乙酸根(TFA)阴离子与前体基质中羟基之间的3c-4e键的稳定化和过渡状态 N-进攻/ O-羰基化环化的可行性本质上是由于N原子更强的亲核性以及富电子的双齿膦配体与底物之间重要的π-π相互作用。此外,这些计算提出了一种非常规的还原消除机理,用于从Pd(II)转变为Pd(0),其中N / O原子对羰基C原子的分子内亲核攻击导致了形式的还原消除产物。Pd(TFA)的计算得出的总势垒为14.8 kcal / mol 其中N / O原子对羰基C原子的分子内亲核攻击导致形成正式的还原消除产物。Pd(TFA)的计算得出的总势垒为14.8 kcal / mol 其中N / O原子对羰基C原子的分子内亲核攻击导致形成正式的还原消除产物。Pd(TFA)的计算得出的总势垒为14.8 kcal / mol2与二齿配位体的氮和23.9千卡/摩尔为钯(OTF)2与齿膦配体是与温和的实验条件下定性地一致。
更新日期:2020-03-19
中文翻译:

二苯乙炔衍生物对吲哚[3,2- c ]香豆素和苯并呋喃[3,2- c ]喹啉酮的钯(II)-配体-配体协同区域发散理论研究
2-羟基-2'-氨基-二苯基乙炔衍生物具有两个不同的活性位点,可通过O-攻击/ N-羰基化环化反应生成苯并呋喃[3,2- c ]喹啉酮,或通过C-羟基提供吲哚并[3,2- c ]香豆素。 N攻击/O-羰基化环化。这项工作提出了基于密度泛函理论的计算研究,以了解钯(II)催化的二苯乙炔衍生物的区域发散反应性的机理和起源。结果表明,该反应具有钯(II)-抗衡离子-配体协同催化作用。O-攻击/ N-羰基化环化主要得益于刚性吸电子双齿氮配体的感应作用以及三氟乙酸根(TFA)阴离子与前体基质中羟基之间的3c-4e键的稳定化和过渡状态 N-进攻/ O-羰基化环化的可行性本质上是由于N原子更强的亲核性以及富电子的双齿膦配体与底物之间重要的π-π相互作用。此外,这些计算提出了一种非常规的还原消除机理,用于从Pd(II)转变为Pd(0),其中N / O原子对羰基C原子的分子内亲核攻击导致了形式的还原消除产物。Pd(TFA)的计算得出的总势垒为14.8 kcal / mol 其中N / O原子对羰基C原子的分子内亲核攻击导致形成正式的还原消除产物。Pd(TFA)的计算得出的总势垒为14.8 kcal / mol 其中N / O原子对羰基C原子的分子内亲核攻击导致形成正式的还原消除产物。Pd(TFA)的计算得出的总势垒为14.8 kcal / mol2与二齿配位体的氮和23.9千卡/摩尔为钯(OTF)2与齿膦配体是与温和的实验条件下定性地一致。





























 京公网安备 11010802027423号
京公网安备 11010802027423号