当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic Ring-Opening Polymerization of N-Acylated-1,4-oxazepan-7-ones Toward Well-Defined Poly(ester amide)s: Biodegradable Alternatives to Poly(2-oxazoline)s
ACS Macro Letters ( IF 5.1 ) Pub Date : 2020-03-18 , DOI: 10.1021/acsmacrolett.0c00040 Xin Wang 1 , Nikos Hadjichristidis 1
ACS Macro Letters ( IF 5.1 ) Pub Date : 2020-03-18 , DOI: 10.1021/acsmacrolett.0c00040 Xin Wang 1 , Nikos Hadjichristidis 1
Affiliation
|
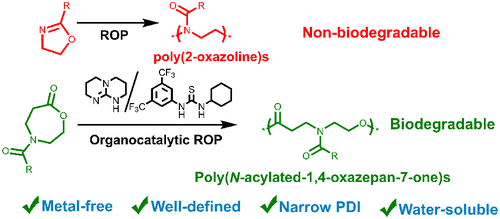
We report a series of poly(ester amide)s (PEAs) synthesized by organocatalytic ring-opening polymerization (ROP) of N-acylated-1,4-oxazepan-7-one (OxP) monomers, produced from N-acylated-4-piperidones using the Baeyer–Villiger oxidation reaction. The ROP of OxPs, conducted in CH2Cl2 at room temperature with benzyl alcohol as initiator and TBD/TU (1,5,7-triazabicyclo[4.4.0]dec-5-ene/thiourea) as a binary organocatalytic system, revealed a controlled/living character. The thermodynamics of the ROP highly depends on the N-acylated substituent of monomers, with the following reactivity order: OxPPh > OxPMe > OxPPr > OxPBn. Based on NMR results, it seems that our system follows the hydrogen bonding bifunctional activation mechanism. All intermediates and final products were characterized by NMR, MALDI-TOF MS, SEC, and DSC techniques. All poly(N-acylated-1,4-oxazepan-7-one) (POxP) polymers are amorphous with different glass transition temperatures (Tg), depending on the N-acylated substituent (Tg: −2.90 to 43.75 °C). Among the synthesized polymers, only POxPMe was water-soluble and it degraded much faster than polycaprolactone in an aqueous phosphate buffer saline solution (pH = 7.4). Therefore, poly(N-acylated-1,4-oxazepan-7-one)s are potential biodegradable alternatives to poly(2-oxazoline)s.
中文翻译:

N-Acylated-1,4-oxazepan-7-ones 的有机催化开环聚合制备明确的聚(酯酰胺):聚(2-恶唑啉)的可生物降解替代品
我们报告了一系列由N -acylated-1,4-oxazepan-7-one (OxP) 单体的有机催化开环聚合 (ROP) 合成的聚 (酯酰胺) (PEA ),由N -acylated-4生产-哌啶酮使用 Baeyer-Villiger 氧化反应。OxPs 的 ROP,在室温下在 CH 2 Cl 2中进行,苯甲醇作为引发剂,TBD/TU (1,5,7-triazabicyclo[4.4.0]dec-5-ene/thiourea) 作为二元有机催化体系,揭示了一个受控/活生生的角色。ROP的热力学高度取决于单体的N-酰化取代基,具有以下反应顺序:OxP Ph > OxP Me > OxP Pr > OxP Bn. 基于核磁共振结果,我们的系统似乎遵循氢键双功能活化机制。所有中间体和最终产品均通过 NMR、MALDI-TOF MS、SEC 和 DSC 技术进行了表征。所有聚(N-酰化-1,4-oxazepan-7-one)(POxP)聚合物都是无定形的,具有不同的玻璃化转变温度(Tg),取决于N-酰化取代基(Tg : -2.90至 43.75 °C ) )。在合成的聚合物中,只有 POxP Me是水溶性的,它在磷酸盐缓冲盐水溶液(pH = 7.4)中的降解速度比聚己内酯快得多。因此,聚(N-acylated-1,4-oxazepan-7-one)s 是聚 (2-oxazoline)s 的潜在可生物降解替代品。
更新日期:2020-04-23
中文翻译:

N-Acylated-1,4-oxazepan-7-ones 的有机催化开环聚合制备明确的聚(酯酰胺):聚(2-恶唑啉)的可生物降解替代品
我们报告了一系列由N -acylated-1,4-oxazepan-7-one (OxP) 单体的有机催化开环聚合 (ROP) 合成的聚 (酯酰胺) (PEA ),由N -acylated-4生产-哌啶酮使用 Baeyer-Villiger 氧化反应。OxPs 的 ROP,在室温下在 CH 2 Cl 2中进行,苯甲醇作为引发剂,TBD/TU (1,5,7-triazabicyclo[4.4.0]dec-5-ene/thiourea) 作为二元有机催化体系,揭示了一个受控/活生生的角色。ROP的热力学高度取决于单体的N-酰化取代基,具有以下反应顺序:OxP Ph > OxP Me > OxP Pr > OxP Bn. 基于核磁共振结果,我们的系统似乎遵循氢键双功能活化机制。所有中间体和最终产品均通过 NMR、MALDI-TOF MS、SEC 和 DSC 技术进行了表征。所有聚(N-酰化-1,4-oxazepan-7-one)(POxP)聚合物都是无定形的,具有不同的玻璃化转变温度(Tg),取决于N-酰化取代基(Tg : -2.90至 43.75 °C ) )。在合成的聚合物中,只有 POxP Me是水溶性的,它在磷酸盐缓冲盐水溶液(pH = 7.4)中的降解速度比聚己内酯快得多。因此,聚(N-acylated-1,4-oxazepan-7-one)s 是聚 (2-oxazoline)s 的潜在可生物降解替代品。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号