当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of Crown‐Ether‐Functionalized Polysulfone Membrane by In Situ Surface Grafting for Selective Adsorption and Separation of Li+
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-03-17 , DOI: 10.1002/slct.201904836 Jixue Li 1, 2 , Hong Yi 3 , Mingxia Wang 1, 4 , Feng Yan 1, 5 , Quanji Zhu 1, 4 , Shouhe Wang 1, 2 , Jianxin Li 1, 4 , Benqiao He 1, 4 , Zhenyu Cui 1, 4
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-03-17 , DOI: 10.1002/slct.201904836 Jixue Li 1, 2 , Hong Yi 3 , Mingxia Wang 1, 4 , Feng Yan 1, 5 , Quanji Zhu 1, 4 , Shouhe Wang 1, 2 , Jianxin Li 1, 4 , Benqiao He 1, 4 , Zhenyu Cui 1, 4
Affiliation
|
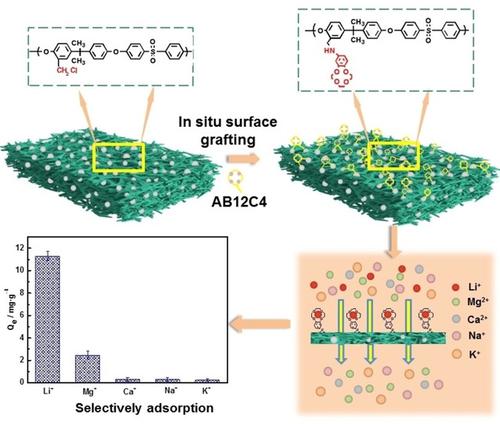
In order to develop an effective and environment‐friendly method for selective adsorption of Li+, a recyclable polysulfone surface graft 4‘‐aminobenzo‐12‐crown‐4‐ether (PSf‐sg‐AB12 C4) membrane was prepared via non‐solvent induced phase separation method and in situ surface grafting through nucleophilic substitution. The surface chemical compositions and the morphologies of the PSf‐sg‐AB12 C4 were systematically verified by X‐ray photoelectron spectra (XPS) and scanning electron microscopy (SEM). The dynamic adsorption process of PSf‐sg‐AB12 C4 for Li+ was well described by the pseudo‐second‐order kinetics model, and the maximum equilibrium adsorption capability was 51.99 mg⋅g‐1. Moreover, the PSf‐sg‐AB12 C4 membrane displayed good selectivity for Li+, meanwhile the selective separation factors (α) of Li+ to K+, Na+, Ca2+ and Mg2+ were 38.43, 33.13, 27.11 and 3.42, respectively. These selective adsorption results were verified by the density functional theory (DFT) calculations. Additionally, the regenerated adsorption amount of the PSf‐sg‐AB12 C4 toward Li+ after 10 cycles of adsorption/desorption was 90.37% of initial adsorption cycle.
中文翻译:

原位表面接枝制备冠醚功能化聚砜膜,用于Li +的选择性吸附和分离
为了开发一种有效和环保的Li +选择性吸附方法,通过非溶剂制备了可回收的聚砜表面接枝4'-氨基苯并-12-皇冠-4-醚(PSf-sg-AB12 C4)膜诱导相分离法和通过亲核取代原位表面接枝。PSf-sg-AB12 C4的表面化学组成和形态已通过X射线光电子能谱(XPS)和扫描电子显微镜(SEM)进行了系统验证。伪二级动力学模型很好地描述了PSf-sg-AB12 C4对Li +的动态吸附过程,最大平衡吸附能力为51.99 mg·g -1。此外,PSf-sg-AB12 C4膜对锂具有良好的选择性+,同时Li +对K +,Na +,Ca 2+和Mg 2+的选择性分离因子(α)分别为38.43、33.13、27.11和3.42。这些选择性吸附结果通过密度泛函理论(DFT)计算得到了验证。另外,经过10次吸附/解吸后,PSf -sg-AB12 C4对Li +的再生吸附量为初始吸附循环的90.37%。
更新日期:2020-03-19
中文翻译:

原位表面接枝制备冠醚功能化聚砜膜,用于Li +的选择性吸附和分离
为了开发一种有效和环保的Li +选择性吸附方法,通过非溶剂制备了可回收的聚砜表面接枝4'-氨基苯并-12-皇冠-4-醚(PSf-sg-AB12 C4)膜诱导相分离法和通过亲核取代原位表面接枝。PSf-sg-AB12 C4的表面化学组成和形态已通过X射线光电子能谱(XPS)和扫描电子显微镜(SEM)进行了系统验证。伪二级动力学模型很好地描述了PSf-sg-AB12 C4对Li +的动态吸附过程,最大平衡吸附能力为51.99 mg·g -1。此外,PSf-sg-AB12 C4膜对锂具有良好的选择性+,同时Li +对K +,Na +,Ca 2+和Mg 2+的选择性分离因子(α)分别为38.43、33.13、27.11和3.42。这些选择性吸附结果通过密度泛函理论(DFT)计算得到了验证。另外,经过10次吸附/解吸后,PSf -sg-AB12 C4对Li +的再生吸附量为初始吸附循环的90.37%。

































 京公网安备 11010802027423号
京公网安备 11010802027423号