Chem ( IF 19.1 ) Pub Date : 2020-03-17 , DOI: 10.1016/j.chempr.2020.02.017 Frederik Sandfort , Felix Strieth-Kalthoff , Marius Kühnemund , Christian Beecks , Frank Glorius
|
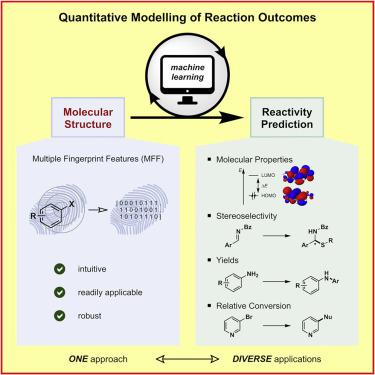
Despite their enormous potential, machine learning methods have only found limited application in predicting reaction outcomes, because current models are often highly complex and, most importantly, are not transferable to different problem sets. Here, we present a structure-based machine learning platform for diverse applications in organic chemistry. Therefore, an input based on multiple fingerprint features (MFFs) as a versatile molecular representation was developed that was shown to be applicable over a range of diverse problem sets. First, molecular properties across a diverse array of molecules could be predicted accurately. Next, reaction outcomes such as stereoselectivities and yields were predicted for experimental datasets that were previously evaluated using (complex) problem-oriented descriptor models. As a final application, a systematic high-throughput dataset was investigated as a “real-world problem,” and good correlation was observed when using the structure-based model.
中文翻译:

基于结构的化学反应性预测平台
尽管机器学习方法具有巨大的潜力,但由于其当前的模型通常非常复杂,并且最重要的是无法转移到不同的问题集,因此机器学习方法在预测反应结果方面的应用非常有限。在这里,我们为有机化学中的各种应用提供了一个基于结构的机器学习平台。因此,开发了基于多种指纹特征(MFF)作为通用分子表示的输入,该输入被证明可应用于一系列不同的问题集。首先,可以准确预测各种分子的分子特性。接下来,针对先前使用(复杂)面向问题的描述符模型进行评估的实验数据集,预测了诸如立体选择性和收率之类的反应结果。作为最终应用,

































 京公网安备 11010802027423号
京公网安备 11010802027423号