Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Equilibria in the Quaternary Systems NaOH + Na2CO3 + Na2SO4 + H2O, Na2CO3 + NaOH + NaCl + H2O, and NaOH + Na2SO4 + NaCl + H2O at 363.15 K
ACS Omega ( IF 3.7 ) Pub Date : 2020-03-09 , DOI: 10.1021/acsomega.9b03703 Jiangman Wu 1 , Jinrong Liu 1 , Zhaojun Wu 1
ACS Omega ( IF 3.7 ) Pub Date : 2020-03-09 , DOI: 10.1021/acsomega.9b03703 Jiangman Wu 1 , Jinrong Liu 1 , Zhaojun Wu 1
Affiliation
|
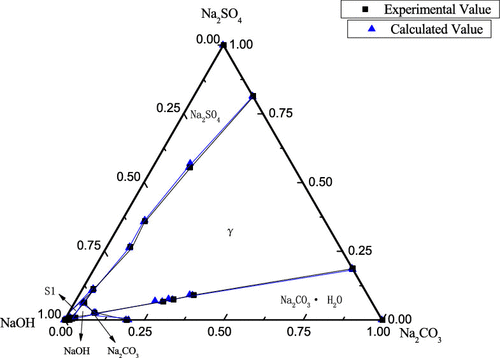
The solid–liquid equilibrium data of the aqueous NaOH–Na2CO3–Na2SO4–H2O, NaOH–Na2CO3–NaCl–H2O, and NaOH–Na2SO4–NaCl–H2O quaternary systems at 363.15 K were measured. The equilibrium solid phases and solubilities of salts in the three systems and its subsystems were determined. The densities of the saturated solutions were also determined. The experimental data are used to plot the solubility diagrams and water content diagrams of the systems. It was found that the NaOH–Na2CO3–Na2SO4–H2O system contains the solid solution of γ-salt (mNa2SO4·nNa2CO3) and the other two systems Na2CO3–NaOH–NaCl–H2O and NaOH–Na2SO4–NaCl–H2O have the complex salts S1 (Na2SO4·NaOH) and S3 (Na2SO4·NaCl·NaOH). On the basis of Xu’s activity coefficient model, a model was constructed for the correlation of solid–liquid equilibrium in electrolyte solutions to calculate the solubilities of salts in these systems at 363.15 K. The calculated solubilities are in agreement with the experimental values.
中文翻译:

四元体系中的相平衡NaOH + Na 2 CO 3 + Na 2 SO 4 + H 2 O,Na 2 CO 3 + NaOH + NaCl + H 2 O和NaOH + Na 2 SO 4 + NaCl + H 2 O在363.15 ķ
NaOH–Na 2 CO 3 –Na 2 SO 4 –H 2 O,NaOH–Na 2 CO 3 –NaCl–H 2 O和NaOH–Na 2 SO 4 –NaCl–H 2水溶液的固液平衡数据测量了363.15 K的O四元系统。确定了这三个系统及其子系统中平衡固相和盐的溶解度。还确定了饱和溶液的密度。实验数据用于绘制系统的溶解度图和水含量图。发现NaOH–Na 2 CO 3 –Na 2SO 4 -H 2 O系统包含γ-盐的固溶体(m Na 2 SO 4 · n Na 2 CO 3)和其他两个系统Na 2 CO 3 -NaOH-NaCl-H 2 O和NaOH-Na 2 SO 4 –NaCl–H 2 O具有复合盐S1(Na 2 SO 4 ·NaOH)和S3(Na 2 SO 4·NaCl·NaOH)。在徐的活度系数模型的基础上,建立了电解质溶液中固液平衡相关性的模型,以计算在363.15 K下这些系统中盐的溶解度。计算的溶解度与实验值一致。
更新日期:2020-03-19
中文翻译:

四元体系中的相平衡NaOH + Na 2 CO 3 + Na 2 SO 4 + H 2 O,Na 2 CO 3 + NaOH + NaCl + H 2 O和NaOH + Na 2 SO 4 + NaCl + H 2 O在363.15 ķ
NaOH–Na 2 CO 3 –Na 2 SO 4 –H 2 O,NaOH–Na 2 CO 3 –NaCl–H 2 O和NaOH–Na 2 SO 4 –NaCl–H 2水溶液的固液平衡数据测量了363.15 K的O四元系统。确定了这三个系统及其子系统中平衡固相和盐的溶解度。还确定了饱和溶液的密度。实验数据用于绘制系统的溶解度图和水含量图。发现NaOH–Na 2 CO 3 –Na 2SO 4 -H 2 O系统包含γ-盐的固溶体(m Na 2 SO 4 · n Na 2 CO 3)和其他两个系统Na 2 CO 3 -NaOH-NaCl-H 2 O和NaOH-Na 2 SO 4 –NaCl–H 2 O具有复合盐S1(Na 2 SO 4 ·NaOH)和S3(Na 2 SO 4·NaCl·NaOH)。在徐的活度系数模型的基础上,建立了电解质溶液中固液平衡相关性的模型,以计算在363.15 K下这些系统中盐的溶解度。计算的溶解度与实验值一致。































 京公网安备 11010802027423号
京公网安备 11010802027423号