当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing CO2 Electroreduction to Ethanol on Copper–Silver Composites by Opening an Alternative Catalytic Pathway
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-16 , DOI: 10.1021/acscatal.9b05319 Louisa Rui Lin Ting 1, 2 , Oriol Piqué 3 , Si Ying Lim 1, 2 , Mohammad Tanhaei 4 , Federico Calle-Vallejo 3 , Boon Siang Yeo 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-16 , DOI: 10.1021/acscatal.9b05319 Louisa Rui Lin Ting 1, 2 , Oriol Piqué 3 , Si Ying Lim 1, 2 , Mohammad Tanhaei 4 , Federico Calle-Vallejo 3 , Boon Siang Yeo 1, 2
Affiliation
|
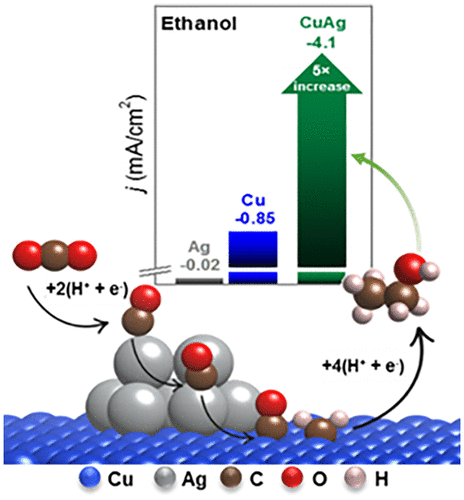
A fundamental question in the electrochemical CO2 reduction reaction (CO2RR) is how to rationally control the catalytic selectivity. For instance, adding a CO-selective cocatalyst like Ag to Cu shifts the latter’s CO2RR selectivity toward C2 products, but the underlying cause of the change is unclear. Herein, we show that, during CO2RR, the abundant CO availability at Cu−Ag boundaries facilitates C-C coupling on Cu to selectively generate ethanol through an otherwise closed pathway. Oxide-derived Cu nanowires mixed with 20 nm Ag particles (Cu:Ag mole ratio of 1:20) catalyzed CO2 reduction to ethanol with a maximum current density of −4.1 mA/cm2 and ethanol/ethylene Faradaic efficiency ratio of 1.1 at −1.1 V vs RHE. These figures of merit are, respectively, 5 and 3 times higher than those for pure oxide-derived Cu nanowires. CO2RR on CuAg composite catalysts with different Ag:Cu ratios and Ag particle sizes reveals that ethanol production scales with the amount of CO evolved from Ag sites and the abundance of Cu–Ag boundaries, and, very interestingly, without significant modifications to ethylene formation. Computational modeling shows selective ethanol generation via Langmuir–Hinshelwood *CO + *CHx (x = 1, 2) coupling at Cu–Ag boundaries and that the formation of energy-intensive CO dimers is circumvented.
中文翻译:

通过开辟另一种催化途径,增强铜银复合材料上乙醇的CO 2电还原
电化学CO 2还原反应(CO 2 RR)中的一个基本问题是如何合理地控制催化选择性。例如,向铜中添加像Ag这样的CO选择性助催化剂会使Cu的CO 2 RR选择性朝C 2产物转移,但是改变的根本原因尚不清楚。在这里,我们表明,在CO 2 RR期间,Cu-Ag边界处的大量CO可用性促进了Cu上的CC偶联,从而通过其他封闭途径选择性地产生乙醇。与20 nm Ag颗粒(Cu:Ag摩尔比为1:20)混合的源自氧化物的Cu纳米线催化将CO 2还原为乙醇,最大电流密度为-4.1 mA / cm 2-1.1 V时的乙醇/乙烯法拉第效率比vs. RHE。这些品质因数分别比纯氧化物衍生的铜纳米线高5到3倍。不同Ag:Cu比和Ag粒径的CuAg复合催化剂上的CO 2 RR表明,乙醇的生产规模随CO的量从Ag位和大量的Cu-Ag边界演化而来,而且非常有趣的是,对乙烯没有明显的修饰编队。计算模型表明,通过Langmuir-Hinshelwood * CO + * CH x(x = 1,2)在Cu-Ag边界上的耦合选择性生成了乙醇,并且避免了高能CO二聚体的形成。
更新日期:2020-03-16
中文翻译:

通过开辟另一种催化途径,增强铜银复合材料上乙醇的CO 2电还原
电化学CO 2还原反应(CO 2 RR)中的一个基本问题是如何合理地控制催化选择性。例如,向铜中添加像Ag这样的CO选择性助催化剂会使Cu的CO 2 RR选择性朝C 2产物转移,但是改变的根本原因尚不清楚。在这里,我们表明,在CO 2 RR期间,Cu-Ag边界处的大量CO可用性促进了Cu上的CC偶联,从而通过其他封闭途径选择性地产生乙醇。与20 nm Ag颗粒(Cu:Ag摩尔比为1:20)混合的源自氧化物的Cu纳米线催化将CO 2还原为乙醇,最大电流密度为-4.1 mA / cm 2-1.1 V时的乙醇/乙烯法拉第效率比vs. RHE。这些品质因数分别比纯氧化物衍生的铜纳米线高5到3倍。不同Ag:Cu比和Ag粒径的CuAg复合催化剂上的CO 2 RR表明,乙醇的生产规模随CO的量从Ag位和大量的Cu-Ag边界演化而来,而且非常有趣的是,对乙烯没有明显的修饰编队。计算模型表明,通过Langmuir-Hinshelwood * CO + * CH x(x = 1,2)在Cu-Ag边界上的耦合选择性生成了乙醇,并且避免了高能CO二聚体的形成。































 京公网安备 11010802027423号
京公网安备 11010802027423号