Nature Communications ( IF 14.7 ) Pub Date : 2020-03-13 , DOI: 10.1038/s41467-020-15184-1 Pascal Albanese 1 , Sem Tamara 2, 3 , Guido Saracco 1 , Richard A Scheltema 2, 3 , Cristina Pagliano 1
|
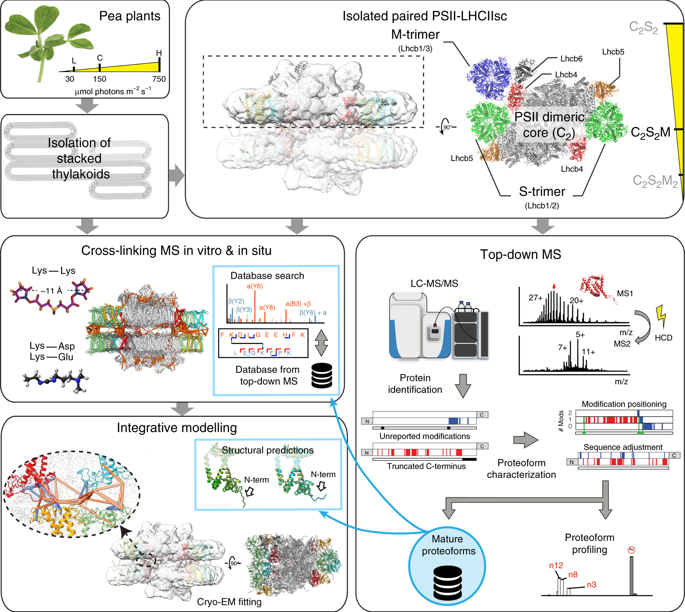
Grana are a characteristic feature of higher plants’ thylakoid membranes, consisting of stacks of appressed membranes enriched in Photosystem II (PSII) and associated light-harvesting complex II (LHCII) proteins, together forming the PSII-LHCII supercomplex. Grana stacks undergo light-dependent structural changes, mainly by reorganizing the supramolecular structure of PSII-LHCII supercomplexes. LHCII is vital for grana formation, in which also PSII-LHCII supercomplexes are involved. By combining top-down and crosslinking mass spectrometry we uncover the spatial organization of paired PSII-LHCII supercomplexes within thylakoid membranes. The resulting model highlights a basic molecular mechanism whereby plants maintain grana stacking at changing light conditions. This mechanism relies on interactions between stroma-exposed N-terminal loops of LHCII trimers and Lhcb4 subunits facing each other in adjacent membranes. The combination of light-dependent LHCII N-terminal trimming and extensive N-terminal α-acetylation likely affects interactions between pairs of PSII-LHCII supercomplexes across the stromal gap, ultimately mediating membrane folding in grana stacks.
中文翻译:

配对的PSII-LHCII超复合物如何介导结构质谱揭示的植物类囊体膜的堆叠。
燕麦是高等植物类囊体膜的特征,由富含光系统II(PSII)和相关的光捕获复合体II(LHCII)蛋白质的贴膜堆叠构成,共同形成PSII-LHCII超复合物。格拉纳叠堆主要通过重组PSII-LHCII超复合物的超分子结构来进行光依赖的结构变化。LHCII对于形成颗粒非常重要,其中还涉及PSII-LHCII超复合物。通过结合自上而下和交联质谱,我们发现类囊体膜内成对的PSII-LHCII超复合物的空间组织。结果模型突出了植物维持谷物的基本分子机制。在变化的光照条件下堆叠。这种机制依赖于LHCII三聚体暴露于基质的N末端环与相邻膜中彼此面对的Lhcb4亚基之间的相互作用。光依赖性LHCII N末端修整和广泛的N末端α-乙酰化的组合可能会影响跨基质间隙的成对PSII-LHCII超复合物之间的相互作用,最终介导了颗粒堆叠中的膜折叠。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号