当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-based design and synthesis of novel furan-diketopiperazine-type derivatives as potent microtubule inhibitors for treating cancer.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-03-13 , DOI: 10.1016/j.bmc.2020.115435 Zhongpeng Ding 1 , Feifei Li 1 , Changjiang Zhong 1 , Feng Li 2 , Yuqian Liu 1 , Shixiao Wang 3 , Jianchun Zhao 4 , Wenbao Li 5
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-03-13 , DOI: 10.1016/j.bmc.2020.115435 Zhongpeng Ding 1 , Feifei Li 1 , Changjiang Zhong 1 , Feng Li 2 , Yuqian Liu 1 , Shixiao Wang 3 , Jianchun Zhao 4 , Wenbao Li 5
Affiliation
|
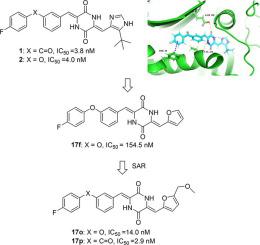
Plinabulin, a synthetic analog of the marine natural product "diketopiperazine phenylahistin," displayed depolymerization effects on microtubules and targeted the colchicine site, which has been moved into phase III clinical trials for the treatment of non-small cell lung cancer (NSCLC) and the prevention of chemotherapy-induced neutropenia (CIN). To develop more potent anti-microtubule and cytotoxic derivatives, the co-crystal complexes of plinabulin derivatives were summarized and analyzed. We performed further modifications of the tert-butyl moiety or C-ring of imidazole-type derivatives to build a library of molecules through the introduction of different groups for novel skeletons. Our structure-activity relationship study indicated that compounds 17o (IC50 = 14.0 nM, NCI-H460) and 17p (IC50 = 2.9 nM, NCI-H460) with furan groups exhibited potent cytotoxic activities at the nanomolar level against various human cancer cell lines. In particular, the 5-methyl or methoxymethyl substituent of furan group could replace the alkyl group of imidazole at the 5-position to maintain cytotoxic activity, contradicting previous reports that the tert-butyl moiety at the 5-position of imidazole was essential for the activity of such compounds. Immunofluorescence assay indicated that compounds 17o and 17p could efficiently inhibit microtubule polymerization. Overall, the novel furan-diketopiperazine-type derivatives could be considered as a potential scaffold for the development of anti-cancer drugs.
中文翻译:

基于结构的设计和合成新型呋喃-二酮哌嗪类衍生物,作为治疗癌症的有效微管抑制剂。
海洋生物天然产物“ diketopiperazine phenylahistin”的合成类似物Plinabulin对微管具有解聚作用,并靶向秋水仙碱位点,秋水仙碱位点已进入III期临床试验,用于治疗非小细胞肺癌(NSCLC)和非小细胞肺癌。预防化疗引起的中性粒细胞减少症(CIN)。为了开发更有效的抗微管和细胞毒性衍生物,总结并分析了纤连蛋白衍生物的共晶体复合物。我们对咪唑型衍生物的叔丁基部分或C环进行了进一步修饰,以通过引入不同骨架的新型骨架建立分子库。我们的结构活性关系研究表明,化合物17o(IC50 = 14.0 nM,NCI-H460)和17p(IC50 = 2.9 nM,具有呋喃基团的NCI-H460)在纳摩尔水平上针对各种人类癌细胞系表现出强力的细胞毒活性。特别是呋喃基团的5-甲基或甲氧基甲基取代基可以取代咪唑在5位上的烷基以维持细胞毒活性,这与以前的报道相反,咪唑5位上的叔丁基部分对于这种化合物的活性。免疫荧光分析表明化合物17o和17p可以有效抑制微管聚合。总体而言,新型呋喃-二酮哌嗪类衍生物可被视为开发抗癌药物的潜在支架。呋喃基团的5-甲基或甲氧基甲基取代基可以取代咪唑在5-位的烷基以维持细胞毒活性,这与以前的报道相反,咪唑5-位的叔丁基部分对于这种活性至关重要。化合物。免疫荧光分析表明化合物17o和17p可以有效抑制微管聚合。总体而言,新型呋喃-二酮哌嗪类衍生物可被视为开发抗癌药物的潜在支架。呋喃基团的5-甲基或甲氧基甲基取代基可以取代咪唑在5-位的烷基以维持细胞毒活性,这与以前的报道相反,咪唑5-位的叔丁基部分对于这种活性至关重要。化合物。免疫荧光分析表明化合物17o和17p可以有效抑制微管聚合。总体而言,新型呋喃-二酮哌嗪类衍生物可被视为开发抗癌药物的潜在支架。免疫荧光分析表明化合物17o和17p可以有效抑制微管聚合。总体而言,新型呋喃-二酮哌嗪类衍生物可被视为开发抗癌药物的潜在支架。免疫荧光分析表明化合物17o和17p可以有效抑制微管聚合。总体而言,新型呋喃-二酮哌嗪类衍生物可被视为开发抗癌药物的潜在支架。
更新日期:2020-04-20
中文翻译:

基于结构的设计和合成新型呋喃-二酮哌嗪类衍生物,作为治疗癌症的有效微管抑制剂。
海洋生物天然产物“ diketopiperazine phenylahistin”的合成类似物Plinabulin对微管具有解聚作用,并靶向秋水仙碱位点,秋水仙碱位点已进入III期临床试验,用于治疗非小细胞肺癌(NSCLC)和非小细胞肺癌。预防化疗引起的中性粒细胞减少症(CIN)。为了开发更有效的抗微管和细胞毒性衍生物,总结并分析了纤连蛋白衍生物的共晶体复合物。我们对咪唑型衍生物的叔丁基部分或C环进行了进一步修饰,以通过引入不同骨架的新型骨架建立分子库。我们的结构活性关系研究表明,化合物17o(IC50 = 14.0 nM,NCI-H460)和17p(IC50 = 2.9 nM,具有呋喃基团的NCI-H460)在纳摩尔水平上针对各种人类癌细胞系表现出强力的细胞毒活性。特别是呋喃基团的5-甲基或甲氧基甲基取代基可以取代咪唑在5位上的烷基以维持细胞毒活性,这与以前的报道相反,咪唑5位上的叔丁基部分对于这种化合物的活性。免疫荧光分析表明化合物17o和17p可以有效抑制微管聚合。总体而言,新型呋喃-二酮哌嗪类衍生物可被视为开发抗癌药物的潜在支架。呋喃基团的5-甲基或甲氧基甲基取代基可以取代咪唑在5-位的烷基以维持细胞毒活性,这与以前的报道相反,咪唑5-位的叔丁基部分对于这种活性至关重要。化合物。免疫荧光分析表明化合物17o和17p可以有效抑制微管聚合。总体而言,新型呋喃-二酮哌嗪类衍生物可被视为开发抗癌药物的潜在支架。呋喃基团的5-甲基或甲氧基甲基取代基可以取代咪唑在5-位的烷基以维持细胞毒活性,这与以前的报道相反,咪唑5-位的叔丁基部分对于这种活性至关重要。化合物。免疫荧光分析表明化合物17o和17p可以有效抑制微管聚合。总体而言,新型呋喃-二酮哌嗪类衍生物可被视为开发抗癌药物的潜在支架。免疫荧光分析表明化合物17o和17p可以有效抑制微管聚合。总体而言,新型呋喃-二酮哌嗪类衍生物可被视为开发抗癌药物的潜在支架。免疫荧光分析表明化合物17o和17p可以有效抑制微管聚合。总体而言,新型呋喃-二酮哌嗪类衍生物可被视为开发抗癌药物的潜在支架。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号