当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Total Synthesis of Cotylenin A
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-13 , DOI: 10.1021/jacs.0c01774 Masahiro Uwamori 1 , Ryunosuke Osada 1 , Ryoji Sugiyama 1 , Kotaro Nagatani 1 , Masahisa Nakada 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-13 , DOI: 10.1021/jacs.0c01774 Masahiro Uwamori 1 , Ryunosuke Osada 1 , Ryoji Sugiyama 1 , Kotaro Nagatani 1 , Masahisa Nakada 1
Affiliation
|
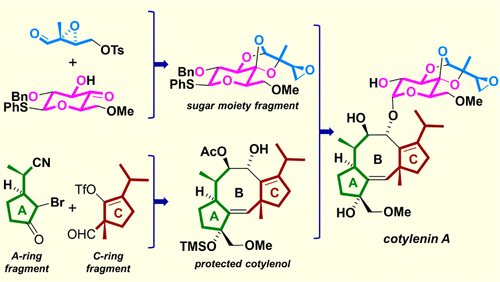
A convergent enantioselective total synthesis of cotylenin A is described. The A-ring fragment, prepared via the catalytic asym-metric intramolecular cyclopropanation developed in our laboratory, and the C-ring fragment, prepared from a known chiral compound via a modified acyl radical cyclization, were successfully assembled by the Utimoto coupling reaction. The formidable carbocyclic eight-membered ring of cotylenin A was efficiently constructed by a palladium-mediated cyclization. All the hydroxy groups in the scaffold were stereoselectively introduced and a modified reducing reagent, Me4NBH(O2CiPr)3, has been developed. The sugar moiety fragment was prepared via three consecutive carbon-oxygen bond-forming reactions, and the glycosylation was accomplished using Wan's protocol.
中文翻译:

Cotylenin A的对映选择性全合成
描述了子叶素 A 的收敛对映选择性全合成。通过我们实验室开发的催化不对称分子内环丙烷化制备的 A 环片段和由已知手性化合物通过修饰的酰基自由基环化制备的 C 环片段通过 Utimoto 偶联反应成功组装。通过钯介导的环化有效地构建了子叶素 A 的强大碳环八元环。支架中的所有羟基都被立体选择性地引入,并开发了一种改良的还原剂 Me4NBH(O2CiPr)3。糖部分片段是通过三个连续的碳氧键形成反应制备的,糖基化是使用万的方案完成的。
更新日期:2020-03-13
中文翻译:

Cotylenin A的对映选择性全合成
描述了子叶素 A 的收敛对映选择性全合成。通过我们实验室开发的催化不对称分子内环丙烷化制备的 A 环片段和由已知手性化合物通过修饰的酰基自由基环化制备的 C 环片段通过 Utimoto 偶联反应成功组装。通过钯介导的环化有效地构建了子叶素 A 的强大碳环八元环。支架中的所有羟基都被立体选择性地引入,并开发了一种改良的还原剂 Me4NBH(O2CiPr)3。糖部分片段是通过三个连续的碳氧键形成反应制备的,糖基化是使用万的方案完成的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号