当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thioxanthone-Based Photobase Generators for the Synthesis of Polyurethanes via the Photopolymerization of Polyols and Polyisocyanates
Macromolecules ( IF 5.1 ) Pub Date : 2020-03-12 , DOI: 10.1021/acs.macromol.9b02648 Nicolas Zivic 1 , Naroa Sadaba 1 , Nora Almandoz 1 , Fernando Ruipérez 1 , David Mecerreyes 1 , Haritz Sardon 1
Macromolecules ( IF 5.1 ) Pub Date : 2020-03-12 , DOI: 10.1021/acs.macromol.9b02648 Nicolas Zivic 1 , Naroa Sadaba 1 , Nora Almandoz 1 , Fernando Ruipérez 1 , David Mecerreyes 1 , Haritz Sardon 1
Affiliation
|
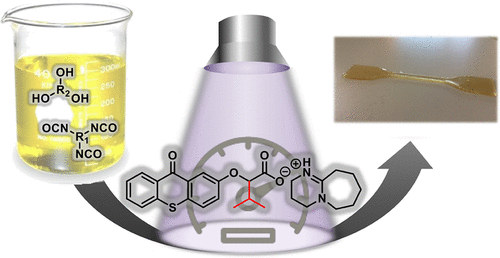
Photopolymerization is a powerful tool in materials science with many applications, including coatings, adhesives, inks, and 3D printing. Until now, the majority of photoinitiating systems have been suitable only for radical photopolymerization, which automatically excludes the use of light to trigger a great number of polymerization reactions. For instance, the preparation of polyurethanes via photopolymerization from isocyanates remains a real challenge since it requires a catalyst able to mediate nucleophilic substitution reactions. In this context, this study reports the successful synthesis of three new photobase generators based on a thioxanthone chromophore functionalized with a protonated 1,8-diazabicyclo[5.4.0]undec-7-ene as a latent base for the direct synthesis of polyurethanes from commercially available polyols and polyisocyanates. The catalytic activity of the photobase is modulated by introducing different functional groups at the α-position of the carboxylate which act as a photocleavable link between the chromophore and the latent base. A direct correlation between the steric hindrance of such groups and more efficient release of the base is observed by 1H NMR. DFT studies have been performed to shed some light on the base release mechanism and to further confirm this evidence. To demonstrate their use, the ability of these photobases to mediate the nucleophilic substitution between isocyanates and alcohols has been proven by using bifunctional and trifunctional monomer mixtures by 1H NMR, FTIR, and rheology experiments. To further exploit the full potential of the thioxanthone-based photobase generators, polyurethane coatings as well as 3D printed figures have been prepared at room temperature by using light as an external trigger.
中文翻译:

基于噻吨酮的光碱发生剂,用于通过多元醇和多异氰酸酯的光聚合来合成聚氨酯
光聚合是材料科学在许多应用中的强大工具,包括涂料,粘合剂,油墨和3D打印。迄今为止,大多数光引发体系仅适用于自由基光聚合,这会自动排除使用光来引发大量聚合反应的可能性。例如,通过光聚合由异氰酸酯制备聚氨酯仍然是一个真正的挑战,因为它需要能够介导亲核取代反应的催化剂。在这种情况下,这项研究报告成功地合成了三种新型的光碱产生剂,其基于以质子化的1,8-二氮杂双环[5.4.0] undec-7-ene官能化的噻吨酮发色团作为直接从中合成聚氨酯的潜在碱市售的多元醇和多异氰酸酯。通过在羧酸的α-位引入不同的官能团来调节光碱的催化活性,所述官能团充当生色团和潜在碱之间的光可裂解的键。通过观察到这些基团的空间位阻与更有效地释放碱之间存在直接关系。1 H NMR。进行了DFT研究以阐明碱基释放机理并进一步证实这一证据。为了证明其用途,通过1 H NMR,FTIR和流变学实验,通过使用双官能和三官能单体混合物,已证明了这些光碱介导异氰酸酯和醇之间亲核取代的能力。为了进一步开发基于噻吨酮的光基发生剂的全部潜能,已在室温下通过使用光作为外部触发器来制备聚氨酯涂料和3D打印图形。
更新日期:2020-03-12
中文翻译:

基于噻吨酮的光碱发生剂,用于通过多元醇和多异氰酸酯的光聚合来合成聚氨酯
光聚合是材料科学在许多应用中的强大工具,包括涂料,粘合剂,油墨和3D打印。迄今为止,大多数光引发体系仅适用于自由基光聚合,这会自动排除使用光来引发大量聚合反应的可能性。例如,通过光聚合由异氰酸酯制备聚氨酯仍然是一个真正的挑战,因为它需要能够介导亲核取代反应的催化剂。在这种情况下,这项研究报告成功地合成了三种新型的光碱产生剂,其基于以质子化的1,8-二氮杂双环[5.4.0] undec-7-ene官能化的噻吨酮发色团作为直接从中合成聚氨酯的潜在碱市售的多元醇和多异氰酸酯。通过在羧酸的α-位引入不同的官能团来调节光碱的催化活性,所述官能团充当生色团和潜在碱之间的光可裂解的键。通过观察到这些基团的空间位阻与更有效地释放碱之间存在直接关系。1 H NMR。进行了DFT研究以阐明碱基释放机理并进一步证实这一证据。为了证明其用途,通过1 H NMR,FTIR和流变学实验,通过使用双官能和三官能单体混合物,已证明了这些光碱介导异氰酸酯和醇之间亲核取代的能力。为了进一步开发基于噻吨酮的光基发生剂的全部潜能,已在室温下通过使用光作为外部触发器来制备聚氨酯涂料和3D打印图形。

































 京公网安备 11010802027423号
京公网安备 11010802027423号