当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical CO oxidation by a Rh tetraaza[14]annulene‐based catalyst
ChemCatChem ( IF 3.8 ) Pub Date : 2020-03-11 , DOI: 10.1002/cctc.202000276 Shin‐ichi Yamazaki 1 , Masafumi Asahi 1 , Zyun Siroma 1 , Tsutomu Ioroi 1
ChemCatChem ( IF 3.8 ) Pub Date : 2020-03-11 , DOI: 10.1002/cctc.202000276 Shin‐ichi Yamazaki 1 , Masafumi Asahi 1 , Zyun Siroma 1 , Tsutomu Ioroi 1
Affiliation
|
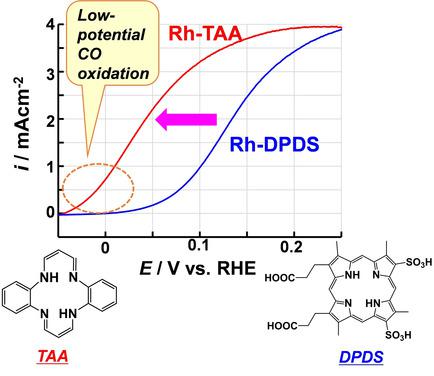
Electrochemical CO oxidation catalyzed by Rh complexes of tetraaza[14]annulene was examined. Rh complexes on carbon black exhibit much higher CO oxidation activity than Rh porphyrins or conventional Pt alloy catalysts. The onset potential for CO oxidation is lower than 0 V vs. a reversible hydrogen electrode. As a result, a combination of electrochemical CO oxidation by Rh tetraaza[14]annulene and proton reduction by Pt catalysts generates slight electricity. The combined overall reaction is a water‐gas shift reaction (CO+H2O→CO2+H2). The co‐presence of Rh tetraaza[14]annulene catalyst and Pt catalyst promotes the water‐gas shift reaction.
中文翻译:

Rh四氮杂[14]环戊烯基催化剂的电化学CO氧化
研究了四氮杂[14]环戊烯的Rh配合物催化的电化学CO氧化。炭黑上的Rh络合物比Rh卟啉或常规Pt合金催化剂具有更高的CO氧化活性。与可逆氢电极相比,CO氧化的起始电位低于0V。结果,Rh四氮杂[14]环戊烯的电化学CO氧化与Pt催化剂的质子还原相结合会产生少量电。总的反应是水煤气变换反应(CO + H 2 O→CO 2 + H 2)。Rh四氮杂[14]环戊烯催化剂和Pt催化剂的共存促进了水煤气变换反应。
更新日期:2020-03-11
中文翻译:

Rh四氮杂[14]环戊烯基催化剂的电化学CO氧化
研究了四氮杂[14]环戊烯的Rh配合物催化的电化学CO氧化。炭黑上的Rh络合物比Rh卟啉或常规Pt合金催化剂具有更高的CO氧化活性。与可逆氢电极相比,CO氧化的起始电位低于0V。结果,Rh四氮杂[14]环戊烯的电化学CO氧化与Pt催化剂的质子还原相结合会产生少量电。总的反应是水煤气变换反应(CO + H 2 O→CO 2 + H 2)。Rh四氮杂[14]环戊烯催化剂和Pt催化剂的共存促进了水煤气变换反应。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号