当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revisiting Indolo[3,2-b]carbazole: Synthesis, Structures, Properties, and Applications.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-03-12 , DOI: 10.1002/anie.202001803 Mengna Zhao 1 , Binghao Zhang 1 , Qian Miao 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-03-12 , DOI: 10.1002/anie.202001803 Mengna Zhao 1 , Binghao Zhang 1 , Qian Miao 1
Affiliation
|
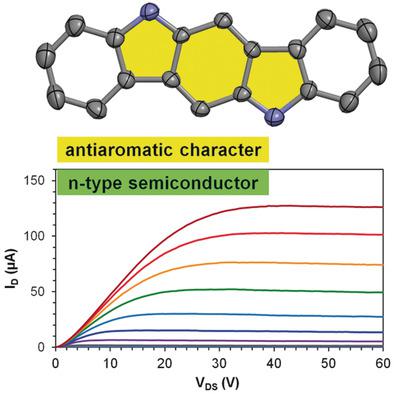
Indolo[3,2‐b ]carbazole presents a π‐skeleton with a remarkable electronic structure and interesting potential applications. It is, however, also associated with ambiguity and controversy. Herein, new derivatives of indolo[3,2‐b ]carbazole are reported and they have enabled a comprehensive study on the electronic structure of indolo[3,2‐b ]carbazole and the development of a new n‐type organic semiconductor. Experimental and computational studies show that indolo[3,2‐b ]carbazole has a largely localized p ‐benzoquinonediimine moiety and significant antiaromaticity. When substituted with (4‐silylethynyl)phenyl groups, the indolo[3,2‐b ]carbazole exhibits one‐dimensional π–π stacking and functions as an n‐type organic semiconductor in solution‐processed field effect transistors.
中文翻译:

吲哚[3,2-b]咔唑的研究:合成,结构,性质和应用。
吲哚[3,2- b ]咔唑呈现出具有杰出的电子结构和有趣的潜在应用的π骨架。但是,它也与模棱两可和争议有关。本文报道了吲哚[3,2- b ]咔唑的新衍生物,它们使吲哚[3,2- b ]咔唑的电子结构和新的n型有机半导体的开发成为可能。实验和计算研究表明,吲哚[3,2- b ]咔唑具有较大的局部对苯二醌二亚胺部分和显着的抗芳香性。当被(4-甲硅烷基乙炔基)苯基取代时,吲哚[3,2- b]咔唑表现出一维π–π堆积,并在溶液处理的场效应晶体管中充当n型有机半导体。
更新日期:2020-03-12
中文翻译:

吲哚[3,2-b]咔唑的研究:合成,结构,性质和应用。
吲哚[3,2- b ]咔唑呈现出具有杰出的电子结构和有趣的潜在应用的π骨架。但是,它也与模棱两可和争议有关。本文报道了吲哚[3,2- b ]咔唑的新衍生物,它们使吲哚[3,2- b ]咔唑的电子结构和新的n型有机半导体的开发成为可能。实验和计算研究表明,吲哚[3,2- b ]咔唑具有较大的局部对苯二醌二亚胺部分和显着的抗芳香性。当被(4-甲硅烷基乙炔基)苯基取代时,吲哚[3,2- b]咔唑表现出一维π–π堆积,并在溶液处理的场效应晶体管中充当n型有机半导体。































 京公网安备 11010802027423号
京公网安备 11010802027423号