当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Near-Complete Suppression of Oxygen Evolution for Photoelectrochemical H2O Oxidative H2O2 Synthesis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-12 , DOI: 10.1021/jacs.9b13410 Kan Zhang 1 , Jiali Liu 1 , Luyang Wang 2 , Bingjun Jin 3 , Xiaofei Yang 4 , Shengli Zhang 1 , Jong Hyeok Park 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-12 , DOI: 10.1021/jacs.9b13410 Kan Zhang 1 , Jiali Liu 1 , Luyang Wang 2 , Bingjun Jin 3 , Xiaofei Yang 4 , Shengli Zhang 1 , Jong Hyeok Park 3
Affiliation
|
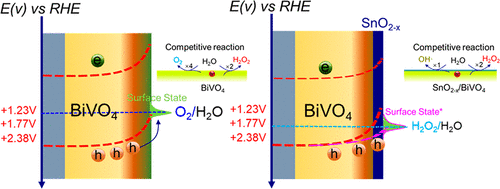
Solar energy-assisted water oxidative hydrogen peroxide (H2O2) production on an anode combined with H2 production on a cathode increases the value of solar water splitting, but the challenge of the dominant oxidative product, O2, needs to be overcome. Here, we report a SnO2-x overlayer coated BiVO4 photoanode, which demonstrates a great abil-ity to near-completely suppress O2 evolution for photoelectrochemical (PEC) H2O oxidative H2O2 evolution. Based on the surface hole accumulation measured by surface photovoltage, downward quasi-hole Fermi energy at the pho-toanode/electrolyte interface and thermodynamic Gibbs free energy between 2-electron and 4-electron competitive reactions, we are able to consider the photoinduced holes of BiVO4 that migrate to the SnO2-x overlayer kinetically favour H2O2 evolution with great selectivity by reduced band bending. Simultaneously, the 1-electron water oxidation reaction is triggered to generate hydroxyl radical (OH) over SnO2-x/BiVO4 photoanode, which, however, is never de-tectable for the BiVO4 photoanode in PEC water splitting. In addition to the H2O oxidative H2O2 evolution from PEC water splitting, the SnO2-x/BiVO4 photoanode can also inhibit H2O2 decomposition into O2 under either electrocataly-sis or photocatalysis conditions for continuous H2O2 accumulation. Overall, the SnO2-x/BiVO4 photoanode achieves a Faraday efficiency (FE) of over 86% for H2O2 generation in a wide potential region (0.6~2.1 V vs. reversible hydrogen electrode (RHE)) and an H2O2 evolution rate averaging 0.825 μmol/min/cm-2 at 1.23 V vs. RHE; this performance sur-passes almost all previous solar energy-assisted H2O2 evolution performances. Because of the simultaneous produc-tion of H2O2 and H2 by solar water splitting in the PEC cells, our results demonstrate a green, cost-effective approach for "solar-to-fuel" conversion.
中文翻译:

近乎完全抑制光电化学 H2O 氧化 H2O2 的析氧
太阳能辅助在阳极上产生水氧化过氧化氢 (H2O2) 与在阴极上产生 H2 相结合,增加了太阳能水分解的价值,但需要克服主要氧化产物 O2 的挑战。在这里,我们报告了一种 SnO2-x 覆盖层涂覆的 BiVO4 光阳极,它展示了几乎完全抑制光电化学 (PEC) H2O 氧化 H2O2 析出的 O2 析出的强大能力。基于通过表面光电压测量的表面空穴积累、光阳极/电解质界面处的向下准空穴费米能和2电子和4电子竞争反应之间的热力学吉布斯自由能,我们能够考虑迁移到 SnO2-x 覆盖层的 BiVO4 的光致空穴在动力学上有利于 H2O2 演化,并且通过减少能带弯曲具有很大的选择性。同时,触发 1 电子水氧化反应以在 SnO2-x/BiVO4 光阳极上产生羟基自由基 (OH),然而,在 PEC 水分解中永远无法检测到 BiVO4 光阳极。除了 PEC 水分解产生的 H2O 氧化 H2O2 外,SnO2-x/BiVO4 光阳极还可以在电催化或光催化条件下抑制 H2O2 分解为 O2,以实现 H2O2 的连续积累。总体而言,SnO2-x/BiVO4 光阳极在宽电位区域(0.6~2.1 V 与可逆氢电极 (RHE))中生成 H2O2 的法拉第效率 (FE) 超过 86%,H2O2 析出率平均为 0。825 μmol/min/cm-2 在 1.23 V vs. RHE;这种性能几乎超越了以前所有的太阳能辅助 H2O2 演化性能。由于在 PEC 电池中通过太阳能水分解同时产生 H2O2 和 H2,我们的结果证明了一种绿色、具有成本效益的“太阳能到燃料”转换方法。
更新日期:2020-03-12
中文翻译:

近乎完全抑制光电化学 H2O 氧化 H2O2 的析氧
太阳能辅助在阳极上产生水氧化过氧化氢 (H2O2) 与在阴极上产生 H2 相结合,增加了太阳能水分解的价值,但需要克服主要氧化产物 O2 的挑战。在这里,我们报告了一种 SnO2-x 覆盖层涂覆的 BiVO4 光阳极,它展示了几乎完全抑制光电化学 (PEC) H2O 氧化 H2O2 析出的 O2 析出的强大能力。基于通过表面光电压测量的表面空穴积累、光阳极/电解质界面处的向下准空穴费米能和2电子和4电子竞争反应之间的热力学吉布斯自由能,我们能够考虑迁移到 SnO2-x 覆盖层的 BiVO4 的光致空穴在动力学上有利于 H2O2 演化,并且通过减少能带弯曲具有很大的选择性。同时,触发 1 电子水氧化反应以在 SnO2-x/BiVO4 光阳极上产生羟基自由基 (OH),然而,在 PEC 水分解中永远无法检测到 BiVO4 光阳极。除了 PEC 水分解产生的 H2O 氧化 H2O2 外,SnO2-x/BiVO4 光阳极还可以在电催化或光催化条件下抑制 H2O2 分解为 O2,以实现 H2O2 的连续积累。总体而言,SnO2-x/BiVO4 光阳极在宽电位区域(0.6~2.1 V 与可逆氢电极 (RHE))中生成 H2O2 的法拉第效率 (FE) 超过 86%,H2O2 析出率平均为 0。825 μmol/min/cm-2 在 1.23 V vs. RHE;这种性能几乎超越了以前所有的太阳能辅助 H2O2 演化性能。由于在 PEC 电池中通过太阳能水分解同时产生 H2O2 和 H2,我们的结果证明了一种绿色、具有成本效益的“太阳能到燃料”转换方法。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号