Scientific Reports ( IF 3.8 ) Pub Date : 2020-03-11 , DOI: 10.1038/s41598-020-61436-x Arkadiusz Gajek 1 , Anastazja Poczta 1 , Małgorzata Łukawska 2 , Violetta Cecuda-Adamczewska 2 , Joanna Tobiasz 2 , Agnieszka Marczak 1
|
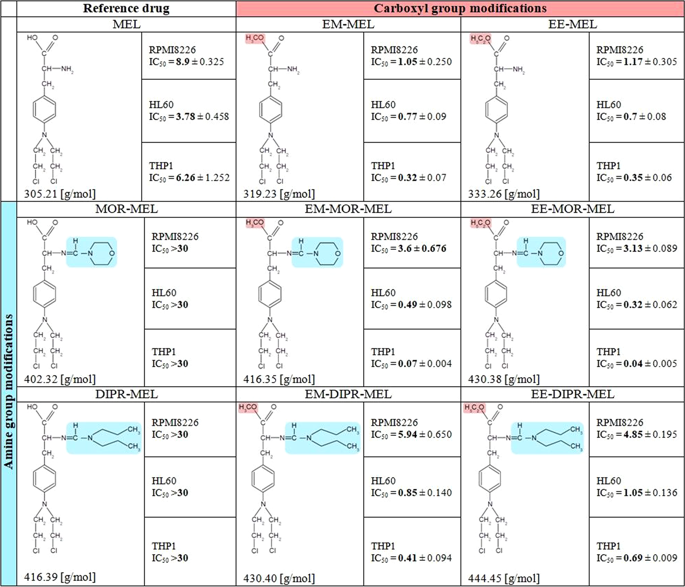
Chemical modification of known, effective drugs is one method to improve chemotherapy. Thus, the object of this study was to generate melphalan derivatives with improved cytotoxic activity in human cancer cells (RPMI8226, HL60 and THP1). Several melphalan derivatives were synthesised, modified in their two important functional groups. Nine analogues were tested, including melphalan compounds modified: only at the amino group, by replacing the amine with an amidine group containing a morpholine ring (MOR-MEL) or with an amidino group and dipropyl chain (DIPR-MEL); only at the carboxyl group to form methyl and ethyl esters of melphalan (EM-MEL, EE-MEL); and in a similar manner at both functional groups (EM-MOR-MEL, EE-MOR-MEL, EM-DIPR-MEL, EE-DIPR-MEL). Melphalan derivatives were evaluated for cytotoxicity (resazurin viability assay), genotoxicity (comet assay) and the ability to induce apoptosis (terminal deoxynucleotidyl transferase dUTP nick end labelling, TUNEL, phosphatidylserine externalisation, chromatin condensation, activity of caspases 3/7, 8 and 9 and intracellular concentration of calcium ions) in comparison with the parent drug. Almost all derivatives, with the exception of MOR-MEL and DIPR-MEL, were found to be more toxic than melphalan in all cell lines evaluated. Treatment of cultures with the derivatives generated a significant higher level of DNA breaks compared to those treated with melphalan, especially after longer incubation times. In addition, all the melphalan derivatives demonstrated a high apoptosis-inducing ability in acute monocytic and promyelocytic leukemia cells. This study showed that the mechanism of action of the tested compounds differed depending on the cell line, and allowed the selection of the most active compounds for further, more detailed investigations.
中文翻译:

美法仑的化学修饰是改善血液系统恶性肿瘤治疗的关键。
已知有效药物的化学修饰是改善化学疗法的一种方法。因此,本研究的目的是在人癌细胞中产生具有改善的细胞毒活性的美法仑衍生物(RPMI8226,HL60和THP1)。合成了几种美法仑衍生物,对其两个重要的官能团进行了修饰。测试了九种类似物,包括被修饰的马法兰化合物:仅在氨基上,通过用含有吗啉环的am基(MOR-MEL)或with基和二丙基链(DIPR-MEL)代替胺;仅在羧基上形成马法兰的甲酯和乙酯(EM-MEL,EE-MEL);并且在两个功能组(EM-MOR-MEL,EE-MOR-MEL,EM-DIPR-MEL,EE-DIPR-MEL)上以类似的方式。评估了Melphalan衍生物的细胞毒性(刃天青活力分析),与亲本相比的遗传毒性(彗星试验)和诱导凋亡的能力(末端脱氧核苷酸转移酶dUTP缺口末端标记,TUNEL,磷脂酰丝氨酸外在化,染色质浓缩,胱天蛋白酶3 / 7、8和9的活性以及细胞内钙离子浓度)药品。在所有评估的细胞系中,除MOR-MEL和DIPR-MEL外,几乎所有衍生物均被发现比马法兰具有更高的毒性。与使用马法兰的处理相比,用衍生物处理的培养物产生的DNA断裂水平高得多,尤其是在较长的孵育时间之后。此外,所有的甲氧苯丙氨酸衍生物在急性单核细胞和早幼粒细胞白血病细胞中均显示出高的细胞凋亡诱导能力。































 京公网安备 11010802027423号
京公网安备 11010802027423号