The Journal of Antibiotics ( IF 2.1 ) Pub Date : 2020-03-11 , DOI: 10.1038/s41429-020-0297-2 Min Zheng 1, 2 , Zhen-Lu Xu 2, 3, 4 , Run-Mei Yang 2 , Shen-Cai Hu 1 , Gang Ding 2 , Jian-Chun Qin 5 , Yong-Gang Zhang 3, 4
|
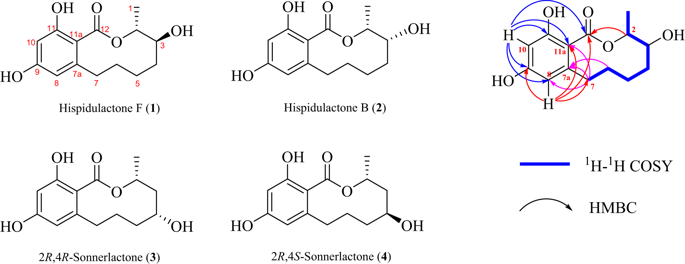
Four 10-membered ring resorcylic acid lactones (RALs) including a new compound hispidulactone F (1) and three known analogs hispidulactone B (2), 2 R, 4R-sonnerlactone (3), and 2 R, 4S-sonnerlactone (4) were isolated from the special bioenvironmental desert plant endophytic fungus Chaetosphaeronema hispidulum. The structure of the new compound hispidulactone F (1) was determined by extensive spectra analysis including HR-ESI-MS, NMR (1H, 13C, 1H-1H COSY, HSQC, and HMBC). Hispidulactone F (1) and hispidulactone B (2) were a pair of stereoisomers at C-3, whereas 2 R, 4R-sonnerlactone (3) and 2 R, 4S-sonnerlactone (4) were another pair of stereoisomers at C-4. The stereochemistries of the hydroxyl groups at C-3 in 1 and 2, and at C-4 in 3 and 4 were first determined by modified Mosher’s reactions. Thus, the absolute configuration C-3 in hispidulactone B (2) was not right in our previous report, and was rectified to be R. Compounds 1 and 4 were evaluated for their cytotoxic effects on the proliferation of HepG2. The possible biosynthetic pathway of compounds 1–4 was also presented.
中文翻译:

立体化学法测定了沙漠植物内生真菌小球藻Chaetosphaeronema hispidulum中的四个10元环间苯二酸内酯。
四个10元环间苯二酸内酯(RAL),包括新的化合物组氨酸内酯F(1)和三个已知的类似物组氨酸内酯B(2),2 R,4 R-松内酯(3)和2 R,4 S-松内酯(4)从特殊的生物沙漠植物内生真菌组脂壳象球藻中分离到。通过包括HR-ESI-MS,NMR(1 H,13 C,1 H- 1在内)的广泛光谱分析确定了新化合物组氨酸内酯F(1)的结构H COSY,HSQC和HMBC)。组氨酸内酯F(1)和组脂内酯B(2)是C-3处的一对立体异构体,而2 R,4 R -sonnerlactone(3)和2 R,4 S -sonnerlactone(4)是C处的另一对立体异构体。 -4。首先通过修饰的Mosher's反应确定1和2中C- 3以及3和4中C-4处的羟基的立体化学。因此,组氨酸内酯B(2)中的绝对构型C-3在我们先前的报告中是不正确的,并且被纠正为R。评价化合物1和4对HepG2增殖的细胞毒性作用。化合物的可能的生物合成途径1 - 4还介绍。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号