Nature ( IF 50.5 ) Pub Date : 2020-03-11 , DOI: 10.1038/s41586-020-2079-1
Qinyang Wang 1 , Yupeng Wang 2, 3 , Jingjin Ding 3, 4 , Chunhong Wang 1 , Xuehan Zhou 1 , Wenqing Gao 3 , Huanwei Huang 3 , Feng Shao 2, 3, 5 , Zhibo Liu 1, 6
|
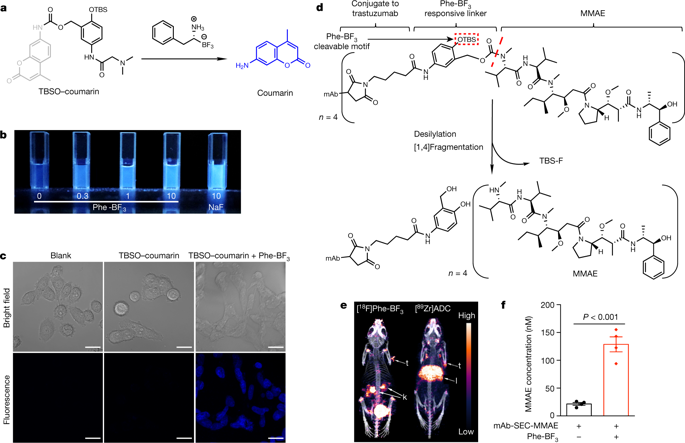
Bioorthogonal chemistry capable of operating in live animals is needed to investigate biological processes such as cell death and immunity. Recent studies have identified a gasdermin family of pore-forming proteins that executes inflammasome-dependent and -independent pyroptosis1,2,3,4,5. Pyroptosis is proinflammatory, but its effect on antitumour immunity is unknown. Here we establish a bioorthogonal chemical system, in which a cancer-imaging probe phenylalanine trifluoroborate (Phe-BF3) that can enter cells desilylates and ‘cleaves’ a designed linker that contains a silyl ether. This system enabled the controlled release of a drug from an antibody–drug conjugate in mice. When combined with nanoparticle-mediated delivery, desilylation catalysed by Phe-BF3 could release a client protein—including an active gasdermin—from a nanoparticle conjugate, selectively into tumour cells in mice. We applied this bioorthogonal system to gasdermin, which revealed that pyroptosis of less than 15% of tumour cells was sufficient to clear the entire 4T1 mammary tumour graft. The tumour regression was absent in immune-deficient mice or upon T cell depletion, and was correlated with augmented antitumour immune responses. The injection of a reduced, ineffective dose of nanoparticle-conjugated gasdermin along with Phe-BF3 sensitized 4T1 tumours to anti-PD1 therapy. Our bioorthogonal system based on Phe-BF3 desilylation is therefore a powerful tool for chemical biology; our application of this system suggests that pyroptosis-induced inflammation triggers robust antitumour immunity and can synergize with checkpoint blockade.
中文翻译:

生物正交系统揭示细胞焦亡的抗肿瘤免疫功能
需要能够在活体动物中操作的生物正交化学来研究细胞死亡和免疫等生物过程。最近的研究已经确定了一个 gasdermin 家族的成孔蛋白,它执行炎性体依赖性和非依赖性细胞焦亡1,2,3,4,5。焦亡是促炎性的,但其对抗肿瘤免疫的影响尚不清楚。在这里,我们建立了一个生物正交化学系统,其中可以进入细胞的癌症成像探针苯丙氨酸三氟硼酸盐 (Phe-BF 3 ) 去甲硅烷基化并“切割”含有甲硅烷基醚的设计接头。该系统能够在小鼠体内控制药物从抗体-药物偶联物中的释放。当与纳米粒子介导的递送相结合时,由 Phe-BF 3催化的去甲硅烷基化可以从纳米颗粒结合物中释放一种客户蛋白——包括一种活性gasdermin——选择性地进入小鼠的肿瘤细胞中。我们将这种生物正交系统应用于gasdermin,结果表明只有不到15%的肿瘤细胞发生焦亡就足以清除整个4T1乳腺肿瘤移植物。免疫缺陷小鼠或 T 细胞耗竭时不存在肿瘤消退,并且与增强的抗肿瘤免疫反应相关。与 Phe-BF 3一起注射减少的、无效剂量的纳米颗粒结合 gasdermin使 4T1 肿瘤对抗 PD1 治疗敏感。我们基于 Phe-BF 3的生物正交系统因此,去甲硅烷化是化学生物学的有力工具;我们对该系统的应用表明,细胞焦亡引起的炎症会触发强大的抗肿瘤免疫,并且可以与检查点封锁协同作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号