Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.cej.2020.124729 Changqing Su , Keke Liu , Junchao Zhu , Hongyu Chen , Hailong Li , Zheng Zeng , Liqing Li
|
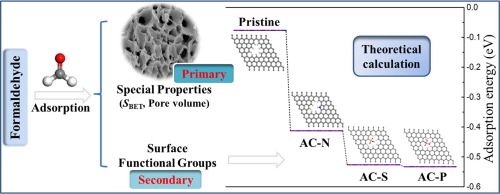
The objectives of this study were to assess the formaldehyde (HCHO) adsorption capacity of doped porous carbon by experiments and theoretical calculations. Nitrogen, sulfur or phosphorus containing porous carbons were prepared with different precursors by activation with KOH at 700 ℃ and compared with commercial activated carbon (CAC), and used as the low HCHO concentration adsorbents in dry (dynamic) and wet (static) conditions. Results showed that the HCHO adsorption capacity of AC-N (2418.20 m2 g-1) for dynamic (13.60 mg g-1) and static (1.43 mg g-1) was greater than that of AC-S, CAC and AC-P. The specific surface area and the micropores volume were expected to markedly influence the adsorption capacity of HCHO. According to the DFT calculation, the adsorption energy of surface functional groups of phosphorus (-0.53296 eV) was the highest, followed by sulfur (-0.52646 eV) and nitrogen (-0.41246 eV), which indicated hydrogen bond interaction played an important role in the adsorption. In the GCMC slit model, the size of slit pores was positively correlated with the adsorption amount of HCHO. With the increase of the adsorption pressure, the surface functional groups had little effect on the adsorption of HCHO under the same size of the slit pores. Accordingly, for the high HCHO adsorption capacity, specific surface area and pore volume are the primary determinant, while surface functional group is the secondary determinant. This would provide a useful theoretical reference for the doping of polarized activated carbon to adsorb polar gases.
中文翻译:

氮,硫或磷表面官能团在环境温度下对甲醛的吸附作用:与计算相关的实验
这项研究的目的是通过实验和理论计算来评估掺杂多孔碳对甲醛的吸附能力。通过在700℃下用KOH活化,用不同的前驱体制备了含氮,硫或磷的多孔碳,并与市售活性炭(CAC)进行了比较,并在干(动态)和湿(静态)条件下用作低HCHO浓度的吸附剂。结果表明,AC-N(2418.20 m 2 g -1)对动态(13.60 mg g -1)和静态(1.43 mg g -1)的HCHO吸附能力)大于AC-S,CAC和AC-P。预期比表面积和微孔体积会显着影响HCHO的吸附能力。根据DFT计算,磷(-0.53296 eV)的表面官能团的吸附能最高,其次是硫(-0.52646 eV)和氮(-0.41246 eV),这表明氢键相互作用在其中起着重要作用。吸附。在GCMC缝隙模型中,缝隙孔的大小与HCHO的吸附量呈正相关。随着吸附压力的增加,在相同孔径的缝隙中,表面官能团对HCHO的吸附影响很小。因此,对于高的HCHO吸附能力,比表面积和孔体积是主要决定因素,表面官能团是次要决定因素。这将为掺杂极化活性炭以吸附极性气体提供有用的理论参考。











































 京公网安备 11010802027423号
京公网安备 11010802027423号