Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced Ferroptosis by Oxygen-Boosted Phototherapy Based on a 2-in-1 Nanoplatform of Ferrous Hemoglobin for Tumor Synergistic Therapy.
ACS Nano ( IF 15.8 ) Pub Date : 2020-03-10 , DOI: 10.1021/acsnano.9b09426 Tian Xu 1 , Yuying Ma 1 , Qinling Yuan 1 , Huixin Hu 1 , Xinkai Hu 1 , Zhiyu Qian 2 , Janiqua Kyiesha Rolle 1 , Yueqing Gu 1 , Siwen Li 1
ACS Nano ( IF 15.8 ) Pub Date : 2020-03-10 , DOI: 10.1021/acsnano.9b09426 Tian Xu 1 , Yuying Ma 1 , Qinling Yuan 1 , Huixin Hu 1 , Xinkai Hu 1 , Zhiyu Qian 2 , Janiqua Kyiesha Rolle 1 , Yueqing Gu 1 , Siwen Li 1
Affiliation
|
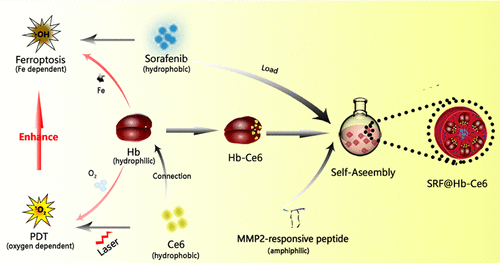
Photodynamic therapy (PDT) combined with oxygenating strategies is widely employed in cancer treatment; however, oxygen-boosted PDT has failed to achieve an ideal effect due to the complexity, heterogeneity, and irreversible hypoxic environment generated by tumor tissues. With the emergence of Fe-dependent ferroptosis boasting reactive oxygen species (ROS) cytotoxicity as well, such a chemodynamic approach to cancer therapy has drawn extensive attention. In this study, hemoglobin (Hb) is connected with the photosensitizer chlorin e6 (Ce6) to construct a 2-in-1 nanoplatform (SRF@Hb-Ce6) with Sorafenib (SRF, ferroptosis promotor) loaded, combining oxygen-boosted PDT and potent ferroptosis. Benefiting from the intrinsic presence of Fe capable of binding oxygen, hemoglobin concurrently furnishes oxygen for oxygen-dependent PDT and Fe for Fe-dependent ferroptosis. Furthermore, amphiphilic MMP2-responsive peptide is incorporated into the skeleton of the nanoplatform to ensure drug-release specificity for safety improvement. Correlative measurements demonstrate the potentiation of PDT and ferroptosis with SRF@Hb-Ce6. More importantly, PDT strengthens ferroptosis by recruiting immune cells to secrete IFN-γ, which can sensitize the tumor to ferroptosis in our findings. The therapeutic effect of synergistic treatment with SRF@Hb-Ce6 in vitro and in vivo was proven significant, revealing the promising prospects of combined PDT and ferroptosis therapy with the 2-in-1 nanoplatform.
中文翻译:

通过基于氧合血红蛋白的二合一纳米平台的氧增强光疗增强肥大症,以进行肿瘤协同治疗。
光动力疗法(PDT)结合氧合策略已广泛用于癌症治疗中。然而,由于肿瘤组织产生的复杂性,异质性和不可逆的缺氧环境,提高氧气的PDT未能达到理想的效果。随着Fe依赖性肥大病的出现,氧还具有活性氧(ROS)的细胞毒性,这种化学疗法用于癌症治疗已引起广泛关注。在这项研究中,将血红蛋白(Hb)与光敏剂二氢卟酚e6(Ce6)连接,以构建载有索拉非尼(SRF,促肥大性启动子)的2合1纳米平台(SRF @ Hb-Ce6),并结合氧气增强的PDT和强效肥大病。受益于能够结合氧的铁的固有存在,血红蛋白同时为氧依赖性PDT提供氧,并为铁依赖性肥大症提供Fe。此外,两亲性MMP2响应肽被整合到纳米平台的骨架中,以确保药物释放的特异性,从而提高安全性。相关的测量结果表明,SRF @ Hb-Ce6增强了PDT和肥大症。更重要的是,PDT通过募集免疫细胞分泌IFN-γ来增强肥大症,在我们的发现中,IFN-γ可以使肿瘤对肥大症敏感。SRF @ Hb-Ce6协同治疗的疗效 更重要的是,PDT通过募集免疫细胞分泌IFN-γ来增强肥大症,在我们的发现中,IFN-γ可以使肿瘤对肥大症敏感。SRF @ Hb-Ce6协同治疗的疗效 更重要的是,PDT通过募集免疫细胞分泌IFN-γ来增强肥大症,在我们的发现中,IFN-γ可以使肿瘤对肥大症敏感。SRF @ Hb-Ce6协同治疗的疗效在体外和体内均被证明具有重要意义,从而揭示了将PDT与铁氧体肥大疗法与二合一纳米平台相结合的广阔前景。
更新日期:2020-03-10
中文翻译:

通过基于氧合血红蛋白的二合一纳米平台的氧增强光疗增强肥大症,以进行肿瘤协同治疗。
光动力疗法(PDT)结合氧合策略已广泛用于癌症治疗中。然而,由于肿瘤组织产生的复杂性,异质性和不可逆的缺氧环境,提高氧气的PDT未能达到理想的效果。随着Fe依赖性肥大病的出现,氧还具有活性氧(ROS)的细胞毒性,这种化学疗法用于癌症治疗已引起广泛关注。在这项研究中,将血红蛋白(Hb)与光敏剂二氢卟酚e6(Ce6)连接,以构建载有索拉非尼(SRF,促肥大性启动子)的2合1纳米平台(SRF @ Hb-Ce6),并结合氧气增强的PDT和强效肥大病。受益于能够结合氧的铁的固有存在,血红蛋白同时为氧依赖性PDT提供氧,并为铁依赖性肥大症提供Fe。此外,两亲性MMP2响应肽被整合到纳米平台的骨架中,以确保药物释放的特异性,从而提高安全性。相关的测量结果表明,SRF @ Hb-Ce6增强了PDT和肥大症。更重要的是,PDT通过募集免疫细胞分泌IFN-γ来增强肥大症,在我们的发现中,IFN-γ可以使肿瘤对肥大症敏感。SRF @ Hb-Ce6协同治疗的疗效 更重要的是,PDT通过募集免疫细胞分泌IFN-γ来增强肥大症,在我们的发现中,IFN-γ可以使肿瘤对肥大症敏感。SRF @ Hb-Ce6协同治疗的疗效 更重要的是,PDT通过募集免疫细胞分泌IFN-γ来增强肥大症,在我们的发现中,IFN-γ可以使肿瘤对肥大症敏感。SRF @ Hb-Ce6协同治疗的疗效在体外和体内均被证明具有重要意义,从而揭示了将PDT与铁氧体肥大疗法与二合一纳米平台相结合的广阔前景。































 京公网安备 11010802027423号
京公网安备 11010802027423号