当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sulfonyl Group Dance: A Tool for the Synthesis of 6-Azido-2-sulfonylpurine Derivatives.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-03-13 , DOI: 10.1021/acs.joc.9b03518 Janis Miķelis Zaķis 1 , Kristers Ozols 1 , Irina Novosjolova 1 , Reinis Vilšķērsts 2, 3 , Anatoly Mishnev 3 , Maris Turks 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-03-13 , DOI: 10.1021/acs.joc.9b03518 Janis Miķelis Zaķis 1 , Kristers Ozols 1 , Irina Novosjolova 1 , Reinis Vilšķērsts 2, 3 , Anatoly Mishnev 3 , Maris Turks 1
Affiliation
|
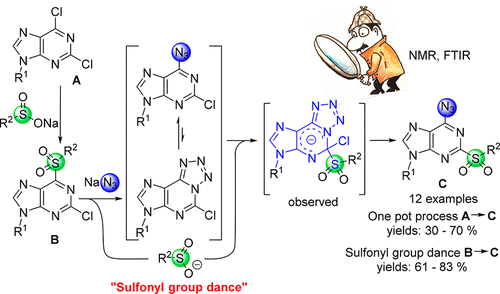
9-Substituted 2-chloro-6-sulfonylpurines provide 6-azido-2-sulfonylpurine derivatives with 61-83% yields when treated with sodium azide. Under optimized reaction conditions, the title compounds are obtained in a one-pot process, which involves a sequential treatment of 2,6-dichloropurines with a selected sodium sulfinate and sodium azide. Such a sulfonyl group dance (functional group swap) results from a cascade of SNAr reactions, which are facilitated by azidoazomethine-tetrazole (azide-tetrazole) tautomeric equilibrium. The formation of Meisenheimer-type intermediates as tetrazolopurine tautomers was supported by various spectroscopic methods, including 15N NMR.
中文翻译:

磺酰基基团舞蹈:合成6-叠氮基-2-磺酰基嘌呤衍生物的工具。
当用叠氮化钠处理时,用9-取代的2-氯-6-磺酰基嘌呤可提供61-83%产率的6-叠氮基-2-磺酰基嘌呤衍生物。在优化的反应条件下,以一锅法获得标题化合物,该过程涉及用选定的亚磺酸钠和叠氮化钠依次处理2,6-二氯嘌呤。磺酰基基团的跳动(官能团交换)是由SNAr反应级联产生的,叠氮基甲亚胺-四唑(叠氮化物-四唑)互变异构平衡促进了这种反应。作为四唑嘌呤互变异构体的迈森海默型中间体的形成受到包括15N NMR在内的各种光谱方法的支持。
更新日期:2020-03-16
中文翻译:

磺酰基基团舞蹈:合成6-叠氮基-2-磺酰基嘌呤衍生物的工具。
当用叠氮化钠处理时,用9-取代的2-氯-6-磺酰基嘌呤可提供61-83%产率的6-叠氮基-2-磺酰基嘌呤衍生物。在优化的反应条件下,以一锅法获得标题化合物,该过程涉及用选定的亚磺酸钠和叠氮化钠依次处理2,6-二氯嘌呤。磺酰基基团的跳动(官能团交换)是由SNAr反应级联产生的,叠氮基甲亚胺-四唑(叠氮化物-四唑)互变异构平衡促进了这种反应。作为四唑嘌呤互变异构体的迈森海默型中间体的形成受到包括15N NMR在内的各种光谱方法的支持。






























 京公网安备 11010802027423号
京公网安备 11010802027423号