当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective catalytic oxidation of ammonia over nano Cu/zeolites with different topologies
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-03-06 , DOI: 10.1039/d0en00007h Hao Wang 1, 2, 3, 4 , Runduo Zhang 1, 2, 3, 4 , Yiyun Liu 5, 6, 7, 8 , Peixin Li 1, 2, 3, 4 , Hongxia Chen 1, 2, 3, 4 , Feng Ryan Wang 5, 6, 7, 8 , Wey Yang Teoh 9, 10, 11, 12
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-03-06 , DOI: 10.1039/d0en00007h Hao Wang 1, 2, 3, 4 , Runduo Zhang 1, 2, 3, 4 , Yiyun Liu 5, 6, 7, 8 , Peixin Li 1, 2, 3, 4 , Hongxia Chen 1, 2, 3, 4 , Feng Ryan Wang 5, 6, 7, 8 , Wey Yang Teoh 9, 10, 11, 12
Affiliation
|
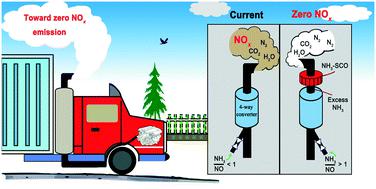
The selective catalytic oxidation of ammonia (NH3-SCO) is the last mitigation step in exhaust treatment using a 4-way catalytic converter to convert any excess and unreacted NH3 (that was used as a reductant of NOx) into environmentally benign N2 and H2O. Here, we report a series of highly reactive and selective nano Cu/zeolites for the NH3-SCO reaction. The NH3-SCO activity was found in the order nano Cu/ZSM-5 (MFI topology) > Cu/Beta (BEA) > Cu/MCM-49 (MWW) > Cu/Y (FAU) > Cu/Mordenite (MOR) > Cu/Ferrierite (FER). The best catalyst, i.e., nano Cu/ZSM-5, achieves 98% NH3 conversion at 250 °C with the N2 yield maintained at >98% even at up to 500 °C. When assessed under practical exhaust conditions in the presence of moisture (5% H2O) as well as that after hydrothermal aging (5% H2O, 850 °C, 8 h), the nano Cu/ZSM-5 exhibited only minor deactivation as a result of its good retention of Cu dispersion, pore structure and specific surface area. Furthermore, small micropore (10-membered ring, 10-MR) topologies were found to be crucial in maintaining high N2 yields. For Cu/Y and Cu/Mordenite, composed of 12-MR pores that are non-interconnected with smaller pores, their N2 yields were compromised by forming NOx at temperatures above 400 °C. Based on the in situ DRIFTS study, the iSCR mechanism appears to be applicable for all fresh and aged Cu/zeolites with the exception of fresh Cu/MCM-49 that follows the imide mechanism.
中文翻译:

具有不同拓扑结构的纳米Cu /沸石上氨的选择性催化氧化
氨的选择性催化氧化(NH 3 -SCO)是排气处理中的最后一个缓解步骤,使用四效催化转化器将任何过量且未反应的NH 3(用作NO x的还原剂)转化为对环境无害的N 2和H 2 O.在此,我们报道了一系列的高反应性和选择性的纳米铜/沸石的NH 3 - SCO反应。NH 3 -SCO活性的发现顺序为:纳米Cu / ZSM-5(MFI拓扑)> Cu /β(BEA)> Cu / MCM-49(MWW)> Cu / Y(FAU)> Cu /丝光沸石(MOR )>铜/镁碱沸石(FER)。最好的催化剂,即纳米Cu / ZSM-5,在250°C的温度下使用N可以达到98%的NH 3转化率。即使在高达500°C的温度下, 2收率仍保持> 98%。当在有水分(5%H 2 O)的情况下以及在水热老化(5%H 2 O,850°C,8小时)的实际排气条件下进行评估时,纳米Cu / ZSM-5仅表现出微量由于其良好的保留铜分散,孔结构和比表面积的结果而失活。此外,发现小的微孔(10元环,10-MR)拓扑对于维持高N 2产量至关重要。对于由不相互连接的12-MR孔和较小孔组成的Cu / Y和Cu / Mordenite,其N 2产率因在高于400°C的温度下形成NO x而受到损害。基于原位 在DRIFTS研究中,iSCR机制似乎适用于所有新鲜和老化的Cu /沸石,但遵循酰亚胺机制的新鲜Cu / MCM-49除外。
更新日期:2020-03-06
中文翻译:

具有不同拓扑结构的纳米Cu /沸石上氨的选择性催化氧化
氨的选择性催化氧化(NH 3 -SCO)是排气处理中的最后一个缓解步骤,使用四效催化转化器将任何过量且未反应的NH 3(用作NO x的还原剂)转化为对环境无害的N 2和H 2 O.在此,我们报道了一系列的高反应性和选择性的纳米铜/沸石的NH 3 - SCO反应。NH 3 -SCO活性的发现顺序为:纳米Cu / ZSM-5(MFI拓扑)> Cu /β(BEA)> Cu / MCM-49(MWW)> Cu / Y(FAU)> Cu /丝光沸石(MOR )>铜/镁碱沸石(FER)。最好的催化剂,即纳米Cu / ZSM-5,在250°C的温度下使用N可以达到98%的NH 3转化率。即使在高达500°C的温度下, 2收率仍保持> 98%。当在有水分(5%H 2 O)的情况下以及在水热老化(5%H 2 O,850°C,8小时)的实际排气条件下进行评估时,纳米Cu / ZSM-5仅表现出微量由于其良好的保留铜分散,孔结构和比表面积的结果而失活。此外,发现小的微孔(10元环,10-MR)拓扑对于维持高N 2产量至关重要。对于由不相互连接的12-MR孔和较小孔组成的Cu / Y和Cu / Mordenite,其N 2产率因在高于400°C的温度下形成NO x而受到损害。基于原位 在DRIFTS研究中,iSCR机制似乎适用于所有新鲜和老化的Cu /沸石,但遵循酰亚胺机制的新鲜Cu / MCM-49除外。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号