Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative assessment of the nature of noncovalent interactions in N-substituted-5-(adamantan-1-yl)-1,3,4-thiadiazole-2-amines: insights from crystallographic and QTAIM analysis
RSC Advances ( IF 3.9 ) Pub Date : 2020-3-6 , DOI: 10.1039/d0ra00733a Ali A El-Emam 1 , Elangovan Saveeth Kumar 2 , Krishnakumar Janani 2 , Lamya H Al-Wahaibi 3 , Olivier Blacque 4 , Mohamed I El-Awady 5 , Nora H Al-Shaalan 3 , M Judith Percino 6 , Subbiah Thamotharan 2
RSC Advances ( IF 3.9 ) Pub Date : 2020-3-6 , DOI: 10.1039/d0ra00733a Ali A El-Emam 1 , Elangovan Saveeth Kumar 2 , Krishnakumar Janani 2 , Lamya H Al-Wahaibi 3 , Olivier Blacque 4 , Mohamed I El-Awady 5 , Nora H Al-Shaalan 3 , M Judith Percino 6 , Subbiah Thamotharan 2
Affiliation
|
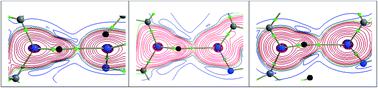
Three adamantane-1,3,4-thiadiazole hybrid derivatives namely; N-ethyl-5-(adamantan-1-yl)-1,3,4-thiadiazole-2-amine I, N-(4-fluorophenyl)-5-(adamantan-1-yl)-1,3,4-thiadiazole-2-amine II and (4-bromophenyl)-5-(adamantan-1-yl)-N-1,3,4-thiadiazole-2-amine III, have been synthesized and crystal structures have been determined at low temperature. The structures revealed that the orientation of the amino group is different in non-halogenated structures. Intra- and intermolecular interactions were characterized on the basis of the quantum theory of atoms-in-molecules (QTAIM) approach. Intermolecular interaction energies for different molecular pairs have been obtained using the PIXEL method. Hirshfeld surface analysis and 2D-fingerprint plots revealed that the relative contributions of different non-covalent interactions are comparable in compounds with halogen (Br and F) substitutions. Crystal structures of II and III show isostructural behaviour with 1D supramolecular constructs. In all three structures, the N–H⋯N hydrogen bond was found to be stronger among other noncovalent interactions. The H–H bonding showed a closed shell in nature and played significant roles in the stabilization of these crystal structures.
中文翻译:

N-取代-5-(金刚烷-1-基)-1,3,4-噻二唑-2-胺中非共价相互作用性质的定量评估:晶体学和 QTAIM 分析的见解
即三金刚烷-1,3,4-噻二唑杂化衍生物;N-乙基-5-(金刚烷-1-基)-1,3,4-噻二唑-2-胺I , N- (4-氟苯基)-5-(金刚烷-1-基)-1,3,4 -噻二唑-2-胺II和 (4-溴苯基)-5-(金刚烷-1-基)-N -1,3,4-噻二唑-2-胺III已在低温下合成并确定了晶体结构温度。结构表明,非卤代结构中氨基的取向是不同的。基于分子内原子量子理论 (QTAIM) 方法来表征分子内和分子间相互作用。使用 PIXEL 方法获得了不同分子对的分子间相互作用能。赫什菲尔德表面分析和二维指纹图表明,在具有卤素(Br 和 F)取代的化合物中,不同非共价相互作用的相对贡献是相当的。II和III的晶体结构显示出与一维超分子结构的同构行为。在所有三种结构中,N-H⋯N 氢键被发现在其他非共价相互作用中更强。H-H 键在自然界中表现出封闭的壳层,并且在稳定这些晶体结构方面发挥着重要作用。
更新日期:2020-03-06
中文翻译:

N-取代-5-(金刚烷-1-基)-1,3,4-噻二唑-2-胺中非共价相互作用性质的定量评估:晶体学和 QTAIM 分析的见解
即三金刚烷-1,3,4-噻二唑杂化衍生物;N-乙基-5-(金刚烷-1-基)-1,3,4-噻二唑-2-胺I , N- (4-氟苯基)-5-(金刚烷-1-基)-1,3,4 -噻二唑-2-胺II和 (4-溴苯基)-5-(金刚烷-1-基)-N -1,3,4-噻二唑-2-胺III已在低温下合成并确定了晶体结构温度。结构表明,非卤代结构中氨基的取向是不同的。基于分子内原子量子理论 (QTAIM) 方法来表征分子内和分子间相互作用。使用 PIXEL 方法获得了不同分子对的分子间相互作用能。赫什菲尔德表面分析和二维指纹图表明,在具有卤素(Br 和 F)取代的化合物中,不同非共价相互作用的相对贡献是相当的。II和III的晶体结构显示出与一维超分子结构的同构行为。在所有三种结构中,N-H⋯N 氢键被发现在其他非共价相互作用中更强。H-H 键在自然界中表现出封闭的壳层,并且在稳定这些晶体结构方面发挥着重要作用。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号