PLOS ONE ( IF 2.9 ) Pub Date : 2020-03-06 , DOI: 10.1371/journal.pone.0229898 Rosa Klotz 1 , Svenja E Seide 2 , Phillip Knebel 1 , Pascal Probst 1 , Thomas Bruckner 2 , Johann Motsch 3 , Alexander Hyhlik-Dürr 4 , Dittmar Böckler 4 , Jan Larmann 3 , Markus K Diener 1 , Markus A Weigand 3 , Markus W Büchler 1 , Andre L Mihaljevic 1
|
Objectives
To test the feasibility of a randomized controlled study design comparing epidural analgesia (EDA) with continuous wound infiltration (CWI) in respect to postoperative complications and mobility to design a future multicentre randomized controlled trial.
Design, setting, participants
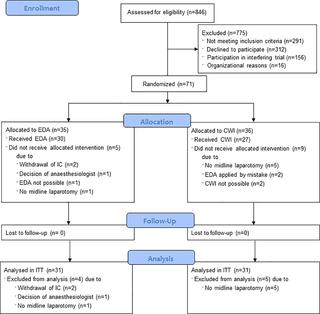
CWI has been developed to address drawbacks of EDA. Previous studies have established the equivalent analgesic potential of CWI compared to EDA. This is a single centre, non-blinded pilot randomized controlled trial at a tertiary surgical centre. Patients undergoing elective non-colorectal surgery via a midline laparotomy were randomized to EDA or CWI. Endpoints included recruitment, feasibility of assessing postoperative mobility with a pedometer and morbidity. No primary endpoint was defined and all analyses were explorative.
Interventions
CWI with local anaesthetics (experimental group) vs. thoracic EDA (control).
Results
Of 846 patients screened within 14 months, 71 were randomized and 62 (31 per group) included in the intention-to-treat analysis. Mobility was assessed in 44 of 62 patients and revealed no differences within the first 3 postoperative days. Overall morbidity did not differ between the two groups (measured via the comprehensive complication index). Median pain scores at rest were comparable between the two groups, while EDA was superior in pain treatment during movement on the first, but not on the second and third postoperative day. Duration of preoperative induction of anaesthesia was shorter with CWI than with EDA. Of 17 serious adverse events, 3 were potentially related to EDA, while none was related to CWI.
Conclusion
This trial confirmed the feasibility of a randomized trial design to compare CWI and EDA regarding morbidity. Improvements in the education and training of team members are necessary to improve recruitment.
Trial registration
DRKS00008023.
中文翻译:

腹部中线切口的连续伤口浸润与硬膜外镇痛 - 一项随机对照试点试验(无痛试点试验;DRKS 编号:DRKS00008023)。
目标
为了测试随机对照研究设计的可行性,比较硬膜外镇痛 (EDA) 与连续伤口浸润 (CWI) 在术后并发症和活动能力方面的可行性,以设计未来的多中心随机对照试验。
设计、设置、参与者
CWI 的开发是为了解决 EDA 的缺点。先前的研究已经确定 CWI 与 EDA 具有同等的镇痛潜力。这是在三级外科中心进行的单中心、非盲式试点随机对照试验。通过中线剖腹手术接受选择性非结直肠手术的患者被随机分配至 EDA 或 CWI。终点包括招募、用计步器评估术后活动能力的可行性和发病率。没有定义主要终点,所有分析都是探索性的。
干预措施
使用局部麻醉剂的 CWI(实验组)与胸部 EDA(对照)。
结果
在 14 个月内筛查的 846 名患者中,71 名患者被随机分配,62 名患者(每组 31 名)纳入意向治疗分析。对 62 名患者中的 44 名进行了活动能力评估,结果显示术后前 3 天内没有差异。两组之间的总体发病率没有差异(通过综合并发症指数测量)。两组之间静息时的中位疼痛评分相当,而 EDA 在术后第一天运动期间的疼痛治疗方面优于,但在术后第二天和第三天则不然。CWI 术前麻醉诱导时间比 EDA 短。在 17 例严重不良事件中,3 例可能与 EDA 有关,而没有一起与 CWI 相关。
结论
该试验证实了比较 CWI 和 EDA 发病率的随机试验设计的可行性。改善团队成员的教育和培训对于改善招聘工作是必要的。
试用注册
DRKS00008023。































 京公网安备 11010802027423号
京公网安备 11010802027423号