Separation and Purification Technology ( IF 8.1 ) Pub Date : 2020-03-06 , DOI: 10.1016/j.seppur.2020.116819 Huan Xiang , Yan Shao , Ahmed Ameen , Huanhao Chen , Weiting Yang , Patricia Gorgojo , Flor R. Siperstein , Xiaolei Fan , Qinhe Pan
|
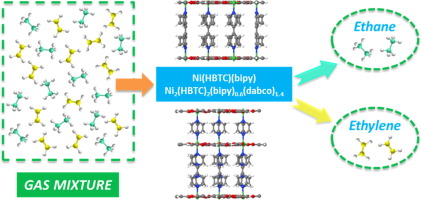
Adsorptive separation is a promising alternative to the energy-intensive cryogenic distillation process for separating the ethane/ethylene (C2H6/C2H4) mixtures. Herein, two pillared-layer metal-organic frameworks (MOFs), Ni(HBTC)(bipy) and Ni2(HBTC)2(bipy)0.6(dabco)1.4, are prepared as the C2H6-selective adsorbents for C2H4 purification from C2H6/C2H4 mixtures. The effect of the type of pillars on the framework structure, thermal and moisture stability, as well as the C2H6 and C2H4 adsorption propertiy, of the MOFs was studied. The use of the longer pillars (i.e. bipy versus dabco) to scafolld the Ni(HBTC) improved the pore size (5.5 Å versus 5.3 Å), specific surface area (1,474 m2/g versus 1,070 m2/g) and moisture stability in the relative humidity range of 0–90%, but compromised the thermal stability (267 °C versus 278 °C). Both MOFs were C2H6-selective, which was evidencd by the single component adsorption experiments using C2H6 and C2H4. The ideal adsorbed solution theory (IAST) selectivity for C2H6/C2H4 mixtures (1:1 and 1:15, v/v) is in the range of 1.4–1.7 at 25–50 °C and 0–1 bar. The preferential adsorption towards C2H6 over C2H4 on both MOFs is then explained by the isosteric heat of adsorption. Additionally, Ni(HBTC)(bipy) also shows the best capacity of up to 6.6 mmol/g for C2H6 adsorption in comparison with other C2H6-selective MOFs at 25 °C and 1 bar. Both MOFs showed the excellent recyclability, with the negligible reduction in the gas uptake observed during four cycles of adsorption/desorption tests. Besides, breakthrough experiments demonstrated that both MOFs can achieve efficient separation of an equimolar C2H6/C2H4 mixture. The findings suggest that Ni(HBTC)(bipy) and Ni2(HBTC)2(bipy)0.6(dabco)1.4 can be further considered as C2H6-trapping adsorbents for the C2H6/C2H4 separation applications in practice.
中文翻译:

C 2 H 6 / C 2 H 4在具有柱状层结构的金属有机骨架(MOF)上的吸附分离
吸附分离是一种用于分离乙烷/乙烯(C 2 H 6 / C 2 H 4)混合物的高能耗低温蒸馏方法的有前途的替代方法。在此,制备两个柱状层金属有机骨架(MOF),即Ni(HBTC)(bipy)和Ni 2(HBTC)2(bipy)0.6(dabco)1.4,作为对C的C 2 H 6选择性吸附剂。2 ħ 4选自C纯化2 ħ 6 / C 2 H ^ 4混合物。研究了支柱类型对MOFs骨架结构,热稳定性和湿气稳定性以及C 2 H 6和C 2 H 4吸附性能的影响。使用时间越长支柱(即联吡啶与DABCO)到scafolld镍(HBTC)改善了孔的尺寸(5.5埃与5.3埃),比表面积(1474米2 / g的与1070米2 / g)和水分稳定性在相对湿度0-90%的范围内,但损害了热稳定性(267°C对278°C)。两个MOF均为C 2 H 6-选择性的,这是通过使用C 2 H 6和C 2 H 4的单组分吸附实验证明的。对于C 2 H 6 / C 2 H 4混合物(1:1和1:15,v / v),理想的吸附溶液理论(IAST)选择性在25–50°C和0–0的范围内为1.4–1.7 1巴。然后通过等规吸附热来解释两个MOF相对于C 2 H 4相对于C 2 H 6的优先吸附。此外,Ni(HBTC)(bipy)对C 2 H的最佳吸附量也高达6.6 mmol / g6吸附在与其它的C比较2 ħ 6在25℃和1巴-选择性的MOF。两种MOF均显示出优异的可回收性,在四个吸附/解吸测试循环中观察到的气体吸收量可忽略不计。此外,突破性实验表明,两种MOF均可实现等摩尔C 2 H 6 / C 2 H 4混合物的有效分离。这一发现表明,镍(HBTC)(联吡啶)和Ni 2(HBTC)2(联吡啶)0.6(DABCO)1.4可以进一步视为c ^ 2 ^ h 6个-trapping吸附剂为C 2H 6 / C 2 H 4分离的实际应用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号