当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal Decomposition Characteristics and Thermal Safety of Dihydroxylammonium 5,5′-Bistetrazole-1,1′-diolate Based on Microcalorimetric Experiment and Decoupling Method
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-03-06 , DOI: 10.1021/acs.jpcc.0c00273 Yabei Xu 1 , Yingxin Tan 1 , Weiguo Cao 1 , Yuxin Zhao 1 , Bin Tian 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-03-06 , DOI: 10.1021/acs.jpcc.0c00273 Yabei Xu 1 , Yingxin Tan 1 , Weiguo Cao 1 , Yuxin Zhao 1 , Bin Tian 1
Affiliation
|
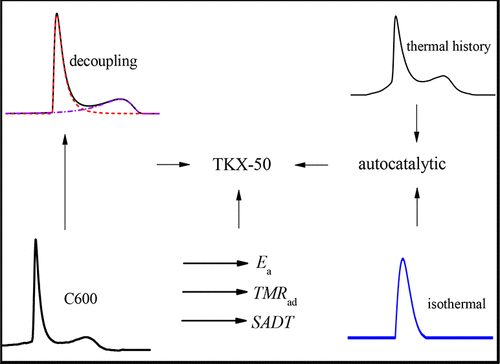
A microcalorimeter (C600) was used to conduct dynamic heating experiments on dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50), and the results were compared with those of differential scanning calorimetry (DSC). The effect of mass scale on the thermal decomposition characteristics of TKX-50 was also analyzed. The thermal decomposition curves were decoupled by mathematical method, and the kinetic parameters of each step were obtained. The thermal decomposition characteristics of TKX-50 were further analyzed by thermal history and isothermal experiment, and the thermal safety parameters were calculated by thermal analysis software (AKTS). The decomposition temperature and decomposition enthalpy of TKX-50 in the microcalorimetric experiment were higher than the corresponding parameters in the DSC experiment, and the apparent activation energy was lower than the one in the DSC experiment. When the times to maximum rate under adiabatic conditions were 2.0, 4.0, 8.0, and 24.0 h, the corresponding temperatures were 198.5, 189.6, 181.0, and 168.0 °C, respectively. After decoupling, the range of exothermic peak temperature and decomposition enthalpy of the first and second stages of TKX-50 were 226.9–245.3 and 276.3–295.7 °C and 1300.7 and 727.7 J g–1, respectively, and the apparent activation energy of the second stage was higher than that of the first stage. The thermal history reduced the decomposition temperature and the apparent activation energy of TKX-50, the safety was reduced, and this had a great influence on the thermal decomposition kinetics of TKX-50. Thermal history and isothermal experiment showed that the first stage decomposition reaction of TKX-50 has autocatalytic properties. Therefore, it should be prevented from being placed in an adiabatic environment, and it is necessary to avoid the storage of a large mass of TKX-50 and keep the heat source off the storage location in the process of industrial production and storage, so as to prevent the formation of adiabatic environments and thermal history in the interior and further reduce the risk of explosion in TKX-50.
中文翻译:

基于微量量热实验和解耦法的5,5'-Bistetrazole-1,1'-glycolate羟基二羟基铵的热分解特性和热安全性
用微量热计(C600)对5,5'-双四唑-1,1'-二醇盐(TKX-50)的二羟基hydroxy进行动态加热实验,并将其结果与差示扫描量热法(DSC)进行比较。还分析了质量尺度对TKX-50热分解特性的影响。用数学方法将热分解曲线解耦,得到各步骤的动力学参数。通过热历史和等温实验进一步分析了TKX-50的热分解特性,并通过热分析软件(AKTS)计算了热安全参数。微量热法实验中TKX-50的分解温度和分解焓高于DSC实验中的相应参数,表观活化能低于DSC实验。在绝热条件下达到最大速率的时间为2.0、4.0、8.0和24.0 h时,相应的温度分别为198.5、189.6、181.0和168.0°C。解耦后,TKX-50第一阶段和第二阶段的放热峰值温度和分解焓的范围为226.9–245.3和276.3–295.7°C和1300.7和727.7 J g–1,第二阶段的表观活化能高于第一阶段。热历史降低了TKX-50的分解温度和表观活化能,降低了安全性,这对TKX-50的热分解动力学有很大影响。热历史和等温实验表明,TKX-50的第一阶段分解反应具有自催化性能。因此,应避免将其放置在绝热环境中,并且在工业生产和存储过程中,有必要避免大量TKX-50的存储,并使热源远离存储位置。以防止内部形成绝热环境和热历史,并进一步降低TKX-50爆炸的风险。
更新日期:2020-03-06
中文翻译:

基于微量量热实验和解耦法的5,5'-Bistetrazole-1,1'-glycolate羟基二羟基铵的热分解特性和热安全性
用微量热计(C600)对5,5'-双四唑-1,1'-二醇盐(TKX-50)的二羟基hydroxy进行动态加热实验,并将其结果与差示扫描量热法(DSC)进行比较。还分析了质量尺度对TKX-50热分解特性的影响。用数学方法将热分解曲线解耦,得到各步骤的动力学参数。通过热历史和等温实验进一步分析了TKX-50的热分解特性,并通过热分析软件(AKTS)计算了热安全参数。微量热法实验中TKX-50的分解温度和分解焓高于DSC实验中的相应参数,表观活化能低于DSC实验。在绝热条件下达到最大速率的时间为2.0、4.0、8.0和24.0 h时,相应的温度分别为198.5、189.6、181.0和168.0°C。解耦后,TKX-50第一阶段和第二阶段的放热峰值温度和分解焓的范围为226.9–245.3和276.3–295.7°C和1300.7和727.7 J g–1,第二阶段的表观活化能高于第一阶段。热历史降低了TKX-50的分解温度和表观活化能,降低了安全性,这对TKX-50的热分解动力学有很大影响。热历史和等温实验表明,TKX-50的第一阶段分解反应具有自催化性能。因此,应避免将其放置在绝热环境中,并且在工业生产和存储过程中,有必要避免大量TKX-50的存储,并使热源远离存储位置。以防止内部形成绝热环境和热历史,并进一步降低TKX-50爆炸的风险。











































 京公网安备 11010802027423号
京公网安备 11010802027423号