当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamics of Transition Metal Ion Binding to Proteins
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-06 , DOI: 10.1021/jacs.0c01329 Lin Frank Song , Arkajyoti Sengupta , Kenneth M. Merz
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-06 , DOI: 10.1021/jacs.0c01329 Lin Frank Song , Arkajyoti Sengupta , Kenneth M. Merz
|
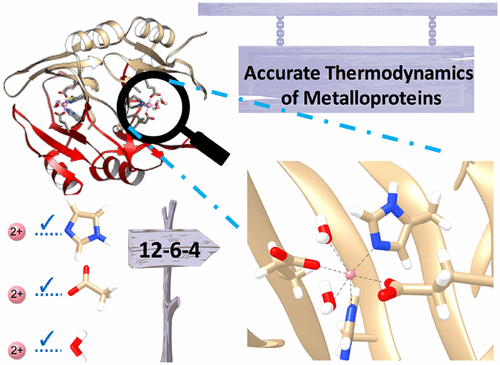
Modeling the thermodynamics of transition metal (TM) ion assembly be it in proteins or in coordination complexes affords us a better understanding of the assembly and function of metalloclusters in diverse application areas including metal organic (MOF) framework design, TM based catalyst design, the trafficking of TM ions in biological systems and drug design in metalloprotein platforms. While the structural details of TM ions bound to metalloproteins are generally well understood via experimental and computational approaches, accurate studies describing the thermodynamics of TM ion binding are rare. Herein, we demonstrate that we can obtain accurate structural and absolute binding free energies of Co2+ and Ni2+ to the enzyme Glyoxalase I (GlxI) using a modified m12-6-4 potential. Critically, this model simultaneously reproduces the solvation free energy of the individual TM ions, reproduces the thermodynamics of TM ion-ligand coordination as well as the thermodynamics of TM ion binding to a protein active site unlike extant models. We find the incorporation of the thermodynamics associated to protonation state changes for the TM ion (un)binding to be crucial. The high accuracy of m12-6-4 potential in this study presents an accurate route to explore more complicated process associated with TM cluster assembly and TM ion transport.
中文翻译:

过渡金属离子与蛋白质结合的热力学
对过渡金属 (TM) 离子组装的热力学进行建模,无论是在蛋白质中还是在配位配合物中,使我们能够更好地了解金属团簇在不同应用领域的组装和功能,包括金属有机 (MOF) 框架设计、基于 TM 的催化剂设计、 TM 离子在生物系统中的运输和金属蛋白平台中的药物设计。虽然通过实验和计算方法通常可以很好地理解 TM 离子与金属蛋白结合的结构细节,但描述 TM 离子结合热力学的准确研究很少见。在此,我们证明我们可以使用修饰的 m12-6-4 电位获得 Co2+ 和 Ni2+ 与酶 Glyoxalase I (GlxI) 的准确结构和绝对结合自由能。关键的是,与现有模型不同,该模型同时再现了单个 TM 离子的溶剂化自由能,再现了 TM 离子-配体配位的热力学以及 TM 离子与蛋白质活性位点结合的热力学。我们发现与 TM 离子(未)结合的质子化状态变化相关的热力学的结合是至关重要的。本研究中 m12-6-4 电位的高精度提供了探索与 TM 簇组装和 TM 离子传输相关的更复杂过程的准确途径。我们发现与 TM 离子(未)结合的质子化状态变化相关的热力学的结合是至关重要的。本研究中 m12-6-4 电位的高精度提供了探索与 TM 簇组装和 TM 离子传输相关的更复杂过程的准确途径。我们发现与 TM 离子(未)结合的质子化状态变化相关的热力学的结合是至关重要的。本研究中 m12-6-4 电位的高精度提供了探索与 TM 簇组装和 TM 离子传输相关的更复杂过程的准确途径。
更新日期:2020-03-06
中文翻译:

过渡金属离子与蛋白质结合的热力学
对过渡金属 (TM) 离子组装的热力学进行建模,无论是在蛋白质中还是在配位配合物中,使我们能够更好地了解金属团簇在不同应用领域的组装和功能,包括金属有机 (MOF) 框架设计、基于 TM 的催化剂设计、 TM 离子在生物系统中的运输和金属蛋白平台中的药物设计。虽然通过实验和计算方法通常可以很好地理解 TM 离子与金属蛋白结合的结构细节,但描述 TM 离子结合热力学的准确研究很少见。在此,我们证明我们可以使用修饰的 m12-6-4 电位获得 Co2+ 和 Ni2+ 与酶 Glyoxalase I (GlxI) 的准确结构和绝对结合自由能。关键的是,与现有模型不同,该模型同时再现了单个 TM 离子的溶剂化自由能,再现了 TM 离子-配体配位的热力学以及 TM 离子与蛋白质活性位点结合的热力学。我们发现与 TM 离子(未)结合的质子化状态变化相关的热力学的结合是至关重要的。本研究中 m12-6-4 电位的高精度提供了探索与 TM 簇组装和 TM 离子传输相关的更复杂过程的准确途径。我们发现与 TM 离子(未)结合的质子化状态变化相关的热力学的结合是至关重要的。本研究中 m12-6-4 电位的高精度提供了探索与 TM 簇组装和 TM 离子传输相关的更复杂过程的准确途径。我们发现与 TM 离子(未)结合的质子化状态变化相关的热力学的结合是至关重要的。本研究中 m12-6-4 电位的高精度提供了探索与 TM 簇组装和 TM 离子传输相关的更复杂过程的准确途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号