Nature ( IF 50.5 ) Pub Date : 2020-03-04 , DOI: 10.1038/s41586-020-2078-2 Xiaoyan Guo 1, 2 , Giovanni Aviles 1, 2 , Yi Liu 3 , Ruilin Tian 1, 2, 4 , Bret A Unger 2, 5 , Yu-Hsiu T Lin 6 , Arun P Wiita 6 , Ke Xu 2, 5 , M Almira Correia 3, 7, 8, 9 , Martin Kampmann 1, 2, 10
|
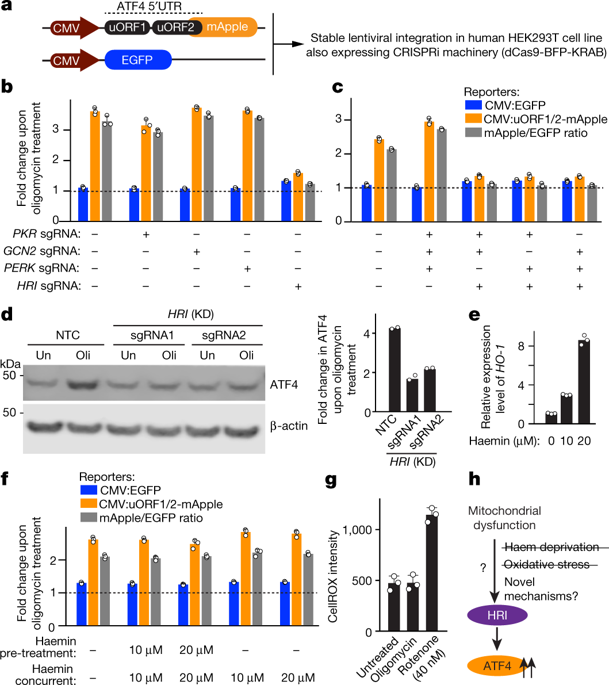
In mammalian cells, mitochondrial dysfunction triggers the integrated stress response, in which the phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) results in the induction of the transcription factor ATF41,2,3. However, how mitochondrial stress is relayed to ATF4 is unknown. Here we show that HRI is the eIF2α kinase that is necessary and sufficient for this relay. In a genome-wide CRISPR interference screen, we identified factors upstream of HRI: OMA1, a mitochondrial stress-activated protease; and DELE1, a little-characterized protein that we found was associated with the inner mitochondrial membrane. Mitochondrial stress stimulates OMA1-dependent cleavage of DELE1 and leads to the accumulation of DELE1 in the cytosol, where it interacts with HRI and activates the eIF2α kinase activity of HRI. In addition, DELE1 is required for ATF4 translation downstream of eIF2α phosphorylation. Blockade of the OMA1–DELE1–HRI pathway triggers an alternative response in which specific molecular chaperones are induced. The OMA1–DELE1–HRI pathway therefore represents a potential therapeutic target that could enable fine-tuning of the integrated stress response for beneficial outcomes in diseases that involve mitochondrial dysfunction.
中文翻译:

线粒体应激通过 OMA1-DELE1-HRI 途径传递到细胞质
在哺乳动物细胞中,线粒体功能障碍触发综合应激反应,其中真核翻译起始因子 2α (eIF2α) 的磷酸化导致转录因子 ATF4 1,2,3的诱导. 然而,线粒体压力如何传递给 ATF4 尚不清楚。在这里,我们表明 HRI 是 eIF2α 激酶,它对于这个中继是必要和充分的。在全基因组 CRISPR 干扰筛选中,我们确定了 HRI 上游的因子:OMA1,一种线粒体应激激活的蛋白酶;和 DELE1,一种我们发现的与线粒体内膜相关的鲜为人知的蛋白质。线粒体应激刺激 DELE1 的 OMA1 依赖性裂解并导致 DELE1 在细胞质中的积累,在那里它与 HRI 相互作用并激活 HRI 的 eIF2α 激酶活性。此外,eIF2α 磷酸化下游的 ATF4 翻译需要 DELE1。OMA1-DELE1-HRI 通路的阻断触发了另一种反应,其中诱导了特定的分子伴侣。































 京公网安备 11010802027423号
京公网安备 11010802027423号