Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-03-04 , DOI: 10.1016/j.bmcl.2020.127076 Hehua Xiong , Jianqing Zhang , Qian Zhang , Yongli Duan , Han Zhang , Pengwu Zheng , Qidong Tang
|
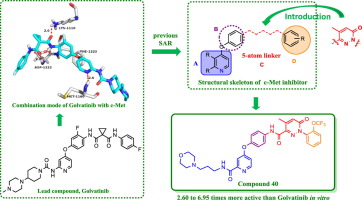
A series of 4-(pyridin-4-yloxy)benzamide derivatives bearing a 5-methylpyridazin-3(2H)-one fragment were designed, synthesized, and evaluated for their biological activity. Most compounds showed effective inhibitory activity against cancer cell lines of A549, HeLa and MCF-7. Among them, the most promising compound 40 showed excellent activity against A549, HeLa and MCF-7 cell lines with IC50 values of 1.03, 1.15 and 2.59 μM, respectively, which was 2.60-6.95 times more active than that of Golvatinib. The structure-activity relationships (SARs) showed that the introduction of 5-methylpyridazin-3(2H)-one to “5-atom linker” and the modification of the amide with morpholine group were beneficial for enhancing the inhibitory activity of compounds. In addition, the further research on compound 40 mainly include c-Met kinase activity, concentration dependence, apoptosis (acridine orange staining), and molecular docking.
中文翻译:

设计,合成和生物学评估的4-(吡啶-4-基氧基)苯甲酰胺衍生物带有一个5-甲基哒嗪-3(2 H)一片段。
设计,合成,评估一系列带有5-甲基吡啶并嗪-3(2 H)一个片段的4-(吡啶-4-基氧基)苯甲酰胺衍生物。大多数化合物显示出对A549,HeLa和MCF-7癌细胞系的有效抑制活性。其中,最有前途的化合物40对A549,HeLa和MCF-7细胞系表现出优异的活性,其IC 50值分别为1.03、1.15和2.59μM,比Golvatinib的活性高2.60-6.95倍。构效关系(SARs)表明5-甲基哒嗪-3(2 H一个至“ 5-原子的连接基”和用吗啉基团修饰酰胺有助于增强化合物的抑制活性。此外,对化合物40的进一步研究主要包括c-Met激酶活性,浓度依赖性,细胞凋亡(ac啶橙染色)和分子对接。































 京公网安备 11010802027423号
京公网安备 11010802027423号