Nano Energy ( IF 16.8 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.nanoen.2020.104669 Junxing Han , Xiaoyi Meng , Liang Lu , Zhong Lin Wang , Chunwen Sun
|
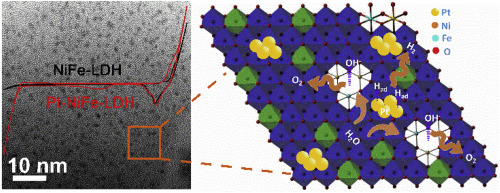
Designing highly efficient electrocatalysts with superior performance on electrochemical water splitting and rechargeable metal-air batteries is an urgent and challenging task due to required large overpotentials. A pulsed direct current with high voltage produced by a layered triboelectric nanogenerator (TENG) provides a novel type of power sources to electrodeposit sub-2 nm Pt nanoclusters onto NiFe-LDH nanosheets in the absence of any capping reagents to improve hydrogen evolution reaction (HER) activity and to electronically reduce part of Fe3+ cations in the lattice of NiFe-LDH to Fe2+ species to enhance oxygen evolution reaction (OER) activity. The particle size of Pt nanoclusters could be easily tuned from 0.8 nm to 1.2 nm by alternating the working frequency of the layered TENG. By loading sub-2 nm Pt nanoclusters onto NiFe-LDH (Pt-NiFe-LDH), the HER overpotential drops from 345 mV to 86 mV at a current density of 50 mA cm-2 in alkaline electrolyte. The synergistic effect between Pt nanoclusters and NiFe-LDH nanosheets on enhancing the cleavage of HO-H bond and recombination of hydrogen intermediates to form molecular hydrogen dramatically improves the HER activity. Meanwhile, benefiting from tuning the local atomic structure and crystal defects by reducing Fe3+ to Fe2+, NiFe-LDH nanosheets demonstrates enhanced OER activity. The deposited sub-2 nm Pt nanoclusters also produces elevated oxygen reduction reaction (ORR) activity with a half-wave potential of 0.84 V (vs RHE). Hence, the as-prepared Pt-NiFe-LDH catalysts exhibits tri-functional properties towards HER, ORR and OER and could be used as the electrode catalysts for overall electrocatalytic water splitting and rechargeable zinc-air batteries. With the optimal catalyst (Pt-NiFe-LDH-0.5-12) as the electrode for overall water splitting, the potential difference between OER and HER drops to 1.63 V at a current density of 50 mA cm-2, much lower than that of the mixed noble-metal catalysts (Pt/C and RuO2, 1.98 V). As an air electrode of rechargeable zinc-air batteries, Pt-NiFe-LDH-0.5-12 exhibits much better performance than that of bare NiFe-LDH in terms of higher open-circuit voltage, higher round-trip efficiency and durability.
中文翻译:

摩擦电纳米发电机驱动的电沉积三功能电催化剂用于水分解和可充电锌空气电池:NiFe-LDH纳米片上的Pt纳米簇
由于需要大的超电势,设计在电化学水分解和可充电金属空气电池上具有卓越性能的高效电催化剂是一项紧迫而艰巨的任务。层状摩擦电纳米发生器(TENG)产生的高压脉冲直流电提供了一种新型电源,可在没有任何封端剂的情况下将亚2 nm Pt纳米簇电沉积到NiFe-LDH纳米片上,以改善氢释放反应(HER活性,并将NiFe-LDH晶格中的部分Fe 3+阳离子电子还原为Fe 2+物种,以增强氧气释放反应(OER)活性。通过改变层状TENG的工作频率,可以很容易地将Pt纳米簇的粒径从0.8 nm调整到1.2 nm。通过将2 nm以下的Pt纳米簇装载到NiFe-LDH(Pt-NiFe-LDH)上,碱性电解液中的电流密度为50 mA cm -2时,HER超电势从345 mV下降到86 mV 。Pt纳米团簇与NiFe-LDH纳米片之间的协同作用对增强HO-H键的裂解和氢中间体的重组形成分子氢的协同作用大大提高了HER活性。同时,通过将Fe 3+还原为Fe 2+受益于调整局部原子结构和晶体缺陷NiFe-LDH纳米片具有增强的OER活性。沉积的亚2 nm Pt纳米簇还产生升高的氧还原反应(ORR)活性,半波电势为0.84 V(vs RHE)。因此,所制备的Pt-NiFe-LDH催化剂表现出对HER,ORR和OER的三官能性质,并且可以用作整个电催化水分解和可充电锌-空气电池的电极催化剂。使用最佳催化剂(Pt-NiFe-LDH-0.5-12)作为总水分解的电极,在50 mA cm -2的电流密度下,OER和HER之间的电势差降至1.63 V ,远低于混合贵金属催化剂(Pt / C和RuO 2,1.98 V)。作为可充电锌空气电池的空气电极,Pt-NiFe-LDH-0.5-12在更高的开路电压,更高的往返效率和耐用性方面表现出比裸NiFe-LDH更好的性能。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号