当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and Electrochemical Impacts of Mg/Mn Dual Dopants on the LiNiO2 Cathode in Li-Metal Batteries.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-03-04 , DOI: 10.1021/acsami.0c00111 Linqin Mu 1 , Wang Hay Kan 2, 3 , Chunguang Kuai 1 , Zhijie Yang 1 , Luxi Li 4 , Cheng-Jun Sun 4 , Sami Sainio 5 , Maxim Avdeev 3, 6 , Dennis Nordlund 5 , Feng Lin 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-03-04 , DOI: 10.1021/acsami.0c00111 Linqin Mu 1 , Wang Hay Kan 2, 3 , Chunguang Kuai 1 , Zhijie Yang 1 , Luxi Li 4 , Cheng-Jun Sun 4 , Sami Sainio 5 , Maxim Avdeev 3, 6 , Dennis Nordlund 5 , Feng Lin 1
Affiliation
|
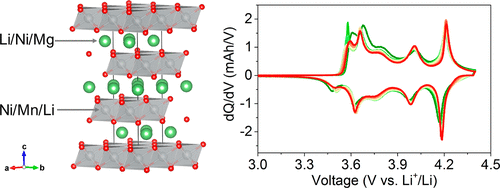
Doping chemistry has been regarded as an efficient strategy to overcome some fundamental challenges facing the "no-cobalt" LiNiO2 cathode materials. By utilizing the doping chemistry, we evaluate the battery performance and structural/chemical reversibility of a new no-cobalt cathode material (Mg/Mn-LiNiO2). The unique dual dopants drive Mg and Mn to occupy the Li site and Ni site, respectively. The Mg/Mn-LiNiO2 cathode delivers smooth voltage profiles, enhanced structural stability, elevated self-discharge resistance, and inhibited nickel dissolution. As a result, the Mg/Mn-LiNiO2 cathode enables improved cycling stability in lithium metal batteries with the conventional carbonate electrolyte: 80% capacity retention after 350 cycles at C/3, and 67% capacity retention after 500 cycles at 2C (22 °C). We then take the Mg/Mn-LiNiO2 as the platform to investigate the local structural and chemical reversibility, where we identify that the irreversibility takes place starting from the very first cycle. The highly reactive surface induces the surface oxygen loss, metal reduction reaching the subsurface, and metal dissolution. Our data demonstrate that the dual dopants can, to some degree, mitigate the irreversibility and improve the cycling stability of LiNiO2, but more efforts are needed to eliminate the key challenges of these materials for battery operation in the conventional carbonate electrolyte.
中文翻译:

Mg / Mn双掺杂对锂金属电池中LiNiO2阴极的结构和电化学影响。
掺杂化学已被认为是克服“无钴” LiNiO2阴极材料所面临的一些基本挑战的有效策略。通过利用掺杂化学,我们评估了新型无钴正极材料(Mg / Mn-LiNiO2)的电池性能和结构/化学可逆性。独特的双重掺杂剂驱动Mg和Mn分别占据Li位和Ni位。Mg / Mn-LiNiO2阴极可提供平滑的电压曲线,增强的结构稳定性,增强的抗自放电性和抑制镍的溶解。结果,Mg / Mn-LiNiO2阴极可改善具有传统碳酸盐电解质的锂金属电池的循环稳定性:在C / 3下350次循环后容量保持80%,在2C(22°C下500次循环后容量保持67% C)。然后,我们以Mg / Mn-LiNiO2为平台,研究局部结构和化学可逆性,从中我们确定不可逆性始于第一个循环。高反应性表面引起表面氧损失,到达地下的金属还原以及金属溶解。我们的数据表明,双重掺杂剂可以在一定程度上减轻LiNiO2的不可逆性并改善其循环稳定性,但是需要更多的努力来消除这些材料在常规碳酸盐电解质中用于电池操作的关键挑战。金属还原到达地下,金属溶解。我们的数据表明,双掺杂剂可以在某种程度上减轻LiNiO2的不可逆性并改善其循环稳定性,但是需要更多的努力来消除这些材料在常规碳酸盐电解质中用于电池操作的关键挑战。金属还原到达地下,金属溶解。我们的数据表明,双掺杂剂可以在某种程度上减轻LiNiO2的不可逆性并改善其循环稳定性,但是需要更多的努力来消除这些材料在常规碳酸盐电解质中用于电池操作的关键挑战。
更新日期:2020-03-05
中文翻译:

Mg / Mn双掺杂对锂金属电池中LiNiO2阴极的结构和电化学影响。
掺杂化学已被认为是克服“无钴” LiNiO2阴极材料所面临的一些基本挑战的有效策略。通过利用掺杂化学,我们评估了新型无钴正极材料(Mg / Mn-LiNiO2)的电池性能和结构/化学可逆性。独特的双重掺杂剂驱动Mg和Mn分别占据Li位和Ni位。Mg / Mn-LiNiO2阴极可提供平滑的电压曲线,增强的结构稳定性,增强的抗自放电性和抑制镍的溶解。结果,Mg / Mn-LiNiO2阴极可改善具有传统碳酸盐电解质的锂金属电池的循环稳定性:在C / 3下350次循环后容量保持80%,在2C(22°C下500次循环后容量保持67% C)。然后,我们以Mg / Mn-LiNiO2为平台,研究局部结构和化学可逆性,从中我们确定不可逆性始于第一个循环。高反应性表面引起表面氧损失,到达地下的金属还原以及金属溶解。我们的数据表明,双重掺杂剂可以在一定程度上减轻LiNiO2的不可逆性并改善其循环稳定性,但是需要更多的努力来消除这些材料在常规碳酸盐电解质中用于电池操作的关键挑战。金属还原到达地下,金属溶解。我们的数据表明,双掺杂剂可以在某种程度上减轻LiNiO2的不可逆性并改善其循环稳定性,但是需要更多的努力来消除这些材料在常规碳酸盐电解质中用于电池操作的关键挑战。金属还原到达地下,金属溶解。我们的数据表明,双掺杂剂可以在某种程度上减轻LiNiO2的不可逆性并改善其循环稳定性,但是需要更多的努力来消除这些材料在常规碳酸盐电解质中用于电池操作的关键挑战。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号