肺癌是全球癌症相关死亡的主要原因。大多数肺癌患者在确诊时已处于晚期,可能会受益于派姆单抗(抗 PD-1 抗体)、细胞毒性化疗和其他辅助治疗。尽管有各种疗法可用,但反应率和存活率一直很低。因此,针对肺癌治疗的不同靶点的研究一直是癌症研究的重点之一。
泛素蛋白酶体系统 (UPS) 是细胞稳态的关键调节剂,在所有细胞的生长发育中起着至关重要的作用。UPS 在包括肺癌细胞在内的人类癌细胞中失调。因此,靶向 UPS 可能是一种选择性、有效的肺癌治疗方法。硼替佐米是一种 20S 蛋白酶体抑制剂,临床上批准用于治疗多发性骨髓瘤,已在各种肺癌的临床前和临床模型中进行了研究。
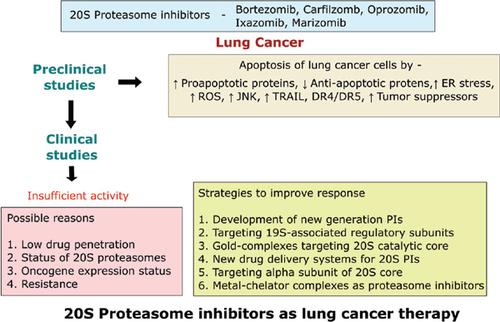
大多数临床前研究表明,单独的 20S 蛋白酶体抑制剂及其与其他化疗药物的组合可诱导非小细胞肺癌细胞系和动物模型的细胞凋亡。由于令人印象深刻的临床前结果,许多临床试验开始使用 20S 蛋白酶体抑制剂作为单一疗法或与其他常规肺癌疗法联合使用。许多 20S PI 与常规化疗的联合疗法在临床试验中显示出良好的耐受性。然而,没有任何一致的数据显示这种基于蛋白酶体抑制剂的疗法的有益效果。20S PIs 在肺癌患者中的低临床疗效可能是由于药物渗透率低、20S 蛋白酶体的状态、癌基因表达以及遗传或获得性耐药。肺癌细胞中 PI 抗性或临床活性低或无临床活性的潜在机制可能包括凋亡蛋白的改变、β5 亚基的过度表达或改变,或热休克蛋白的上调。已经审查了对抗这种耐药性或提高 20S PIs 在肺癌细胞中的功效的各种前沿策略,其中包括新的联合疗法、新的药物递送系统、更有效的 PIs 的开发以及针对 UPS 的不同部位。更好地了解肺癌细胞中的 PI 耐药机制有助于改善当前的临床治疗策略和临床结果。已经审查了对抗这种耐药性或提高 20S PIs 在肺癌细胞中的功效的各种前沿策略,其中包括新的联合疗法、新的药物递送系统、更有效的 PIs 的开发以及针对 UPS 的不同部位。更好地了解肺癌细胞中的 PI 耐药机制有助于改善当前的临床治疗策略和临床结果。已经审查了对抗这种耐药性或提高 20S PIs 在肺癌细胞中的功效的各种前沿策略,其中包括新的联合疗法、新的药物递送系统、更有效的 PIs 的开发以及针对 UPS 的不同部位。更好地了解肺癌细胞中的 PI 耐药机制有助于改善当前的临床治疗策略和临床结果。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Updated Review and Perspective on 20S Proteasome Inhibitors in the Treatment of Lung Cancer.
Lung cancer is the leading cause of cancer-related deaths worldwide. Most lung cancer patients are diagnosed at advanced stages and may benefit from pembrolizumab (anti-PD-1 antibody), cytotoxic chemotherapy and other adjuvant therapies. Despite the availability of various therapies, the response and survival rates have been low. Therefore, the study of different targets for the treatment of lung cancer has been one of the major focuses of cancer research.
The ubiquitin proteasome system (UPS) is a crucial regulator of cell homeostasis and plays an essential role in the growth and development of all cells. The UPS is dysregulated in human cancer cells including lung cancer cells. Therefore, targeting UPS is potentially a selective, effective treatment for lung cancer. Bortezomib, a 20S proteasome inhibitor that is clinically approved for the management of multiple myeloma, has been studied in various preclinical and clinical models of lung cancer.
Most preclinical studies have shown that a 20S proteasome inhibitor alone and its combination with other chemotherapeutic agents induce apoptosis in non-small cell lung cancer cell lines and animal models. Owing to the impressive preclinical results, many clinical trials were initiated using 20S proteasome inhibitors either as monotherapy or in combination with other conventional lung cancer therapies. Many combinational therapies of 20S PIs with conventional chemotherapy were shown to be well tolerated in clinical trials. However, there have not been any consistent data showing the beneficial effects of such proteasome inhibitor-based therapies. Low clinical efficacy of 20S PIs in lung cancer patients may be due to low drug penetration, the status of 20S proteasomes, oncogene expressions and the inherited or acquired resistance. Potential mechanisms of PI resistance or low or no clinical activity in lung cancer cells might include alteration of apoptotic proteins, overexpression or alteration of β5 subunit, or upregulation of heat shock proteins. Various cutting-edge strategies to counter this resistance or improve 20S PIs’ efficacy in lung cancer cells have been reviewed which include novel combination therapies, new drug delivery systems, development of more potent PIs, and targeting different sites of the UPS. A better understanding of PI resistance mechanisms in lung cancer cells can help improve current clinical treatment strategies and clinical outcomes.
































 京公网安备 11010802027423号
京公网安备 11010802027423号