Current Protein & Peptide Science ( IF 1.9 ) Pub Date : 2020-05-31 , DOI: 10.2174/1389203721666200213102829 Tianwen Wang 1 , Ningning Yang 1 , Chen Liang 1 , Hongjv Xu 1 , Yafei An 1 , Sha Xiao 1 , Mengyuan Zheng 1 , Lu Liu 1 , Gaozhan Wang 1 , Lei Nie 1
|
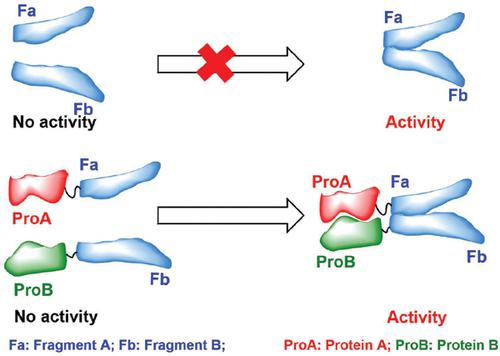
Proteins are the most critical executive molecules by responding to the instructions stored in the genetic materials in any form of life. More frequently, proteins do their jobs by acting as a roleplayer that interacts with other protein(s), which is more evident when the function of a protein is examined in the real context of a cell. Identifying the interactions between (or amongst) proteins is very crucial for the biochemistry investigation of an individual protein and for the attempts aiming to draw a holo-picture for the interacting members at the scale of proteomics (or protein-protein interactions mapping). Here, we introduced the currently available reporting systems that can be used to probe the interaction between candidate protein pairs based on the fragment complementation of some particular proteins. Emphasis was put on the principles and details of experimental design. These systems are dihydrofolate reductase (DHFR), β-lactamase, tobacco etch virus (TEV) protease, luciferase, β- galactosidase, GAL4, horseradish peroxidase (HRP), focal adhesion kinase (FAK), green fluorescent protein (GFP), and ubiquitin.
中文翻译:

基于蛋白质片段互补分析的蛋白质-蛋白质相互作用检测。
蛋白质是对任何生命形式的遗传物质中存储的指令作出响应的最关键的执行分子。蛋白质通常通过充当与其他蛋白质相互作用的角色扮演者来完成其工作,这在细胞的真实环境中检查蛋白质的功能时更为明显。鉴定蛋白质之间(或之中)之间的相互作用对于单个蛋白质的生物化学研究以及旨在以蛋白质组学规模(或蛋白质-蛋白质相互作用图谱)绘制相互作用成员的全貌的尝试而言至关重要。在这里,我们介绍了当前可用的报告系统,该系统可用于基于某些特定蛋白质的片段互补来探查候选蛋白质对之间的相互作用。重点放在实验设计的原理和细节上。这些系统是二氢叶酸还原酶(DHFR),β-内酰胺酶,烟草蚀刻病毒(TEV)蛋白酶,萤光素酶,β-半乳糖苷酶,GAL4,辣根过氧化物酶(HRP),粘着斑激酶(FAK),绿色荧光蛋白(GFP)和泛素。






























 京公网安备 11010802027423号
京公网安备 11010802027423号