当前位置:
X-MOL 学术
›
J. Cell. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
PDK1 is important lipid kinase for RANKL-induced osteoclast formation and function via the regulation of the Akt-GSK3β-NFATc1 signaling cascade.
Journal of Cellular Biochemistry ( IF 3.0 ) Pub Date : 2020-02-12 , DOI: 10.1002/jcb.29677 Dongliang Xiao 1 , Quan Zhou 2 , Yunbing Gao 1 , Baichuan Cao 1 , Qiong Zhang 3 , Gaofeng Zeng 3 , Shaohui Zong 1, 4
Journal of Cellular Biochemistry ( IF 3.0 ) Pub Date : 2020-02-12 , DOI: 10.1002/jcb.29677 Dongliang Xiao 1 , Quan Zhou 2 , Yunbing Gao 1 , Baichuan Cao 1 , Qiong Zhang 3 , Gaofeng Zeng 3 , Shaohui Zong 1, 4
Affiliation
|
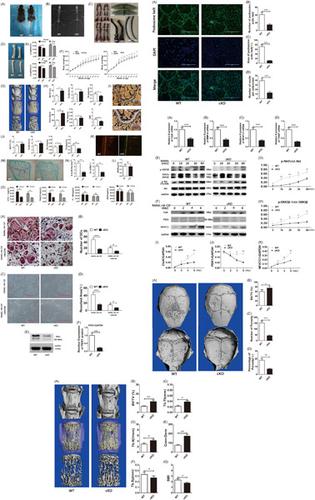
Perturbations in the balanced process of osteoblast‐mediated bone formation and osteoclast‐mediated bone resorption leading to excessive osteoclast formation and/or activity is the cause of many pathological bone conditions such as osteoporosis. The osteoclast is the only cell in the body capable of resorbing and degrading the mineralized bone matrix. Osteoclast formation from monocytic precursors is governed by the actions of two key cytokines macrophage‐colony‐stimulating factor and receptor activator of nuclear factor‐κB ligand (RANKL). Binding of RANKL binding to receptor RANK initiates a series of downstream signaling responses leading to monocytic cell differentiation and fusion, and subsequent mature osteoclast bone resorption and survival. The phosphoinositide‐3‐kinase (PI3K)‐protein kinase B (Akt) signaling cascade is one such pathway activated in response to RANKL. The 3‐phosphoinositide‐dependent protein kinase 1 (PDK1), is considered the master upstream lipid kinase of the PI3K‐Akt cascade. PDK1 functions to phosphorylate and partially activate Akt, triggering the activation of downstream effectors. However, the role of PDK1 in osteoclasts has yet to be clearly defined. In this study, we specifically deleted the PDK1 gene in osteoclasts using the cathepsin‐K promoter driven Cre‐LoxP system. We found that the specific genetic ablation of PDK1 in osteoclasts leads to an osteoclast‐poor osteopetrotic phenotype in mice. In vitro cellular assays further confirmed the impairment of osteoclast formation in response to RANKL by PDK1‐deficient bone marrow macrophage (BMM) precursor cells. PDK1‐deficient BMMs exhibited reduced ability to reorganize actin cytoskeleton to form a podosomal actin belt as a result of diminished capacity to fuse into giant multinucleated osteoclasts. Notably, biochemical analyses showed that PDK1 deficiency attenuated the phosphorylation of Akt and downstream effector GSK3β, and reduced induction of NFATc1. GSK3β is a reported negative regulator of NFATc1. GSK3β activity is inhibited by Akt‐dependent phosphorylation. Thus, our data provide clear genetic and mechanistic insights into the important role for PDK1 in osteoclasts.
中文翻译:

PDK1 是 RANKL 诱导的破骨细胞形成和功能的重要脂质激酶,通过调节 Akt-GSK3β-NFATc1 信号级联。
成骨细胞介导的骨形成和破骨细胞介导的骨吸收平衡过程中的扰动导致破骨细胞过度形成和/或活性是许多病理性骨病如骨质疏松症的原因。破骨细胞是体内唯一能够吸收和降解矿化骨基质的细胞。单核细胞前体的破骨细胞形成受两种关键细胞因子巨噬细胞集落刺激因子和核因子κB配体受体激活剂(RANKL)的作用控制。RANKL 与受体 RANK 的结合启动了一系列下游信号反应,导致单核细胞分化和融合,以及随后成熟的破骨细胞骨吸收和存活。磷酸肌醇 3-激酶 (PI3K)-蛋白激酶 B (Akt) 信号级联反应是响应 RANKL 激活的一种途径。3-磷酸肌醇依赖性蛋白激酶 1 (PDK1) 被认为是 PI3K-Akt 级联的主要上游脂质激酶。PDK1 的功能是磷酸化并部分激活 Akt,从而触发下游效应器的激活。然而,PDK1 在破骨细胞中的作用尚未明确。在本研究中,我们专门删除了使用组织蛋白酶-K 启动子驱动的Cre-LoxP系统检测破骨细胞中的PDK1基因。我们发现破骨细胞中 PDK1 的特定遗传消融导致小鼠的破骨细胞缺乏骨硬化表型。体外细胞测定进一步证实了PDK1缺陷的骨髓巨噬细胞 (BMM) 前体细胞对 RANKL 响应的破骨细胞形成受损。由于融合成巨大多核破骨细胞的能力减弱,PDK1缺陷型 BMM 表现出重组肌动蛋白细胞骨架形成足状肌动蛋白带的能力降低。值得注意的是,生化分析表明PDK1缺乏会减弱 Akt 和下游效应子 GSK3β 的磷酸化,并减少 NFATc1 的诱导。GSK3β 是 NFATc1 的负调节因子。GSK3β 活性受到 Akt 依赖性磷酸化的抑制。因此,我们的数据为 PDK1 在破骨细胞中的重要作用提供了清晰的遗传和机制见解。
更新日期:2020-02-12
中文翻译:

PDK1 是 RANKL 诱导的破骨细胞形成和功能的重要脂质激酶,通过调节 Akt-GSK3β-NFATc1 信号级联。
成骨细胞介导的骨形成和破骨细胞介导的骨吸收平衡过程中的扰动导致破骨细胞过度形成和/或活性是许多病理性骨病如骨质疏松症的原因。破骨细胞是体内唯一能够吸收和降解矿化骨基质的细胞。单核细胞前体的破骨细胞形成受两种关键细胞因子巨噬细胞集落刺激因子和核因子κB配体受体激活剂(RANKL)的作用控制。RANKL 与受体 RANK 的结合启动了一系列下游信号反应,导致单核细胞分化和融合,以及随后成熟的破骨细胞骨吸收和存活。磷酸肌醇 3-激酶 (PI3K)-蛋白激酶 B (Akt) 信号级联反应是响应 RANKL 激活的一种途径。3-磷酸肌醇依赖性蛋白激酶 1 (PDK1) 被认为是 PI3K-Akt 级联的主要上游脂质激酶。PDK1 的功能是磷酸化并部分激活 Akt,从而触发下游效应器的激活。然而,PDK1 在破骨细胞中的作用尚未明确。在本研究中,我们专门删除了使用组织蛋白酶-K 启动子驱动的Cre-LoxP系统检测破骨细胞中的PDK1基因。我们发现破骨细胞中 PDK1 的特定遗传消融导致小鼠的破骨细胞缺乏骨硬化表型。体外细胞测定进一步证实了PDK1缺陷的骨髓巨噬细胞 (BMM) 前体细胞对 RANKL 响应的破骨细胞形成受损。由于融合成巨大多核破骨细胞的能力减弱,PDK1缺陷型 BMM 表现出重组肌动蛋白细胞骨架形成足状肌动蛋白带的能力降低。值得注意的是,生化分析表明PDK1缺乏会减弱 Akt 和下游效应子 GSK3β 的磷酸化,并减少 NFATc1 的诱导。GSK3β 是 NFATc1 的负调节因子。GSK3β 活性受到 Akt 依赖性磷酸化的抑制。因此,我们的数据为 PDK1 在破骨细胞中的重要作用提供了清晰的遗传和机制见解。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号