当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of 3-(3-(4-(1-Aminocyclobutyl)phenyl)-5-phenyl-3H-imidazo[4,5-b]pyridin-2-yl)pyridin-2-amine (ARQ 092): An Orally Bioavailable, Selective, and Potent Allosteric AKT Inhibitor
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2016-06-29 00:00:00 , DOI: 10.1021/acs.jmedchem.6b00619 Jean-Marc Lapierre 1 , Sudharshan Eathiraj 1 , David Vensel 1 , Yanbin Liu 1 , Cathy O. Bull 1 , Susan Cornell-Kennon 1 , Shin Iimura 2 , Eugene W. Kelleher 1 , Darin E. Kizer 1 , Steffi Koerner 1 , Sapna Makhija 1 , Akihisa Matsuda 2 , Magdi Moussa 1 , Nivedita Namdev 1 , Ronald E. Savage 1 , Jeff Szwaya 1 , Erika Volckova 1 , Neil Westlund 1 , Hui Wu 1 , Brian Schwartz 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2016-06-29 00:00:00 , DOI: 10.1021/acs.jmedchem.6b00619 Jean-Marc Lapierre 1 , Sudharshan Eathiraj 1 , David Vensel 1 , Yanbin Liu 1 , Cathy O. Bull 1 , Susan Cornell-Kennon 1 , Shin Iimura 2 , Eugene W. Kelleher 1 , Darin E. Kizer 1 , Steffi Koerner 1 , Sapna Makhija 1 , Akihisa Matsuda 2 , Magdi Moussa 1 , Nivedita Namdev 1 , Ronald E. Savage 1 , Jeff Szwaya 1 , Erika Volckova 1 , Neil Westlund 1 , Hui Wu 1 , Brian Schwartz 1
Affiliation
|
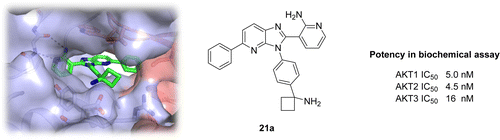
The work in this paper describes the optimization of the 3-(3-phenyl-3H-imidazo[4,5-b]pyridin-2-yl)pyridin-2-amine chemical series as potent, selective allosteric inhibitors of AKT kinases, leading to the discovery of ARQ 092 (21a). The cocrystal structure of compound 21a bound to full-length AKT1 confirmed the allosteric mode of inhibition of this chemical class and the role of the cyclobutylamine moiety. Compound 21a demonstrated high enzymatic potency against AKT1, AKT2, and AKT3, as well as potent cellular inhibition of AKT activation and the phosphorylation of the downstream target PRAS40. Compound 21a also served as a potent inhibitor of the AKT1-E17K mutant protein and inhibited tumor growth in a human xenograft mouse model of endometrial adenocarcinoma.
中文翻译:

发现3-(3-(4-(1-氨基环丁基)苯基)-5-苯基-3 H-咪唑并[4,5 - b ]吡啶-2-基)吡啶-2-胺(ARQ 092)口服生物利用性,选择性和强效变构性AKT抑制剂
本文的工作描述了3-(3-苯基-3 H-咪唑并[4,5 - b ]吡啶-2-基)吡啶-2-胺化学系列的优化,作为AKT激酶的有效,选择性变构抑制剂,导致发现了ARQ 092(21a)。与全长AKT1结合的化合物21a的共晶体结构证实了该化学类别的变构抑制模式以及环丁胺部分的作用。化合物21a对AKT1,AKT2和AKT3具有很高的酶促效力,并且对AKT活化和下游靶标PRAS40的磷酸化具有强大的细胞抑制作用。化合物21a 它还在人子宫内膜腺癌异种移植小鼠模型中用作AKT1-E17K突变蛋白的有效抑制剂,并抑制肿瘤生长。
更新日期:2016-06-29
中文翻译:

发现3-(3-(4-(1-氨基环丁基)苯基)-5-苯基-3 H-咪唑并[4,5 - b ]吡啶-2-基)吡啶-2-胺(ARQ 092)口服生物利用性,选择性和强效变构性AKT抑制剂
本文的工作描述了3-(3-苯基-3 H-咪唑并[4,5 - b ]吡啶-2-基)吡啶-2-胺化学系列的优化,作为AKT激酶的有效,选择性变构抑制剂,导致发现了ARQ 092(21a)。与全长AKT1结合的化合物21a的共晶体结构证实了该化学类别的变构抑制模式以及环丁胺部分的作用。化合物21a对AKT1,AKT2和AKT3具有很高的酶促效力,并且对AKT活化和下游靶标PRAS40的磷酸化具有强大的细胞抑制作用。化合物21a 它还在人子宫内膜腺癌异种移植小鼠模型中用作AKT1-E17K突变蛋白的有效抑制剂,并抑制肿瘤生长。






























 京公网安备 11010802027423号
京公网安备 11010802027423号