当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of (R)-3-(tert-Butoxycarbonylamino)-4-(2,4,5-trifluorophenyl)butanoic Acid, a Key Intermediate, and the Formal Synthesis of Sitagliptin Phosphate.
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-04-20 , DOI: 10.1002/asia.202000092 Kurella Sreenivasulu 1 , Pramod S Chaudhari 1 , Srinivas Achanta 1 , Abhishek Sud 1 , Vilas Dahanukar 1 , Christopher J Cobley 2 , Fiona Llewellyn-Beard 2 , Rakeshwar Bandichhor 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-04-20 , DOI: 10.1002/asia.202000092 Kurella Sreenivasulu 1 , Pramod S Chaudhari 1 , Srinivas Achanta 1 , Abhishek Sud 1 , Vilas Dahanukar 1 , Christopher J Cobley 2 , Fiona Llewellyn-Beard 2 , Rakeshwar Bandichhor 1
Affiliation
|
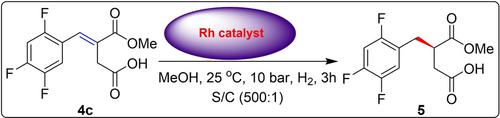
An alternate formal synthesis of Sitagliptin phosphate is disclosed from 2,4,5-trifluorobenzadehyde in 8 linear steps with an overall yield of 31%. The chiral β-amino acid moiety present in sitaglitpin is installed via an asymmetric hydrogenation followed by a stereoselective Hofmann rearrangement as the key steps. The key chiral intermediate Boc-amino acid 1 prepared by this novel route was further converted to Sitagliptin phosphate following the known literature protocol.
中文翻译:

关键中间体(R)-3-(叔丁氧羰基氨基)-4-(2,4,5-三氟苯基)丁酸的合成和磷酸西他列汀的形式合成。
由2,4,5-三氟苯甲醛以8个线性步骤公开了磷酸西他列汀的替代形式合成,总产率为31%。西他立肽中存在的手性β-氨基酸部分是通过不对称氢化,随后进行立体选择性霍夫曼重排而安装的,这是关键步骤。按照已知的文献方法,通过该新途径制备的关键手性中间体Boc-氨基酸1被进一步转化为磷酸西他列汀。
更新日期:2020-03-03
中文翻译:

关键中间体(R)-3-(叔丁氧羰基氨基)-4-(2,4,5-三氟苯基)丁酸的合成和磷酸西他列汀的形式合成。
由2,4,5-三氟苯甲醛以8个线性步骤公开了磷酸西他列汀的替代形式合成,总产率为31%。西他立肽中存在的手性β-氨基酸部分是通过不对称氢化,随后进行立体选择性霍夫曼重排而安装的,这是关键步骤。按照已知的文献方法,通过该新途径制备的关键手性中间体Boc-氨基酸1被进一步转化为磷酸西他列汀。






























 京公网安备 11010802027423号
京公网安备 11010802027423号