当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereoselective Total Synthesis of (‐)‐Ebelactone A
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-03-04 , DOI: 10.1002/slct.201904949 Khandregula Srinivasu 1, 2 , Kommu Nagaiah 1 , Jhillu S. Yadav 1, 2, 3
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-03-04 , DOI: 10.1002/slct.201904949 Khandregula Srinivasu 1, 2 , Kommu Nagaiah 1 , Jhillu S. Yadav 1, 2, 3
Affiliation
|
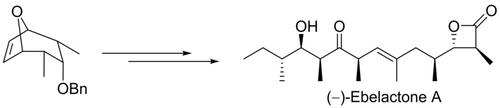
A highly stereoselective total synthesis of the β‐lactam inhibitor of several therapeutically important enzymes, (‐)‐ebelactone A (1) is described. The salient features of this synthesis include desymmetrization of bicyclic symmetric olefin with Brown's asymmetric hydroboration, Wittig olefination, Evans′ alkylation, Sharpless asymmetric epoxidation, Gillman's reaction, TEMPO‐BAIB mediated selective oxidation of 1,3‐diol and Adam's β‐lactonization.
中文翻译:

(‐)‐ Ebelactone A的立体选择性全合成
描述了几种治疗上重要的酶(-)-belactone A(1)的β-内酰胺抑制剂的高度立体选择性全合成。该合成的显着特征包括双环对称烯烃的不对称化与布朗的不对称硼氢化,维蒂希烯化,埃文斯烷基化,夏普莱斯不对称环氧化,吉尔曼反应,TEMPO-BAIB介导的1,3-二醇的选择性氧化和亚当的β-内酯化。
更新日期:2020-03-04
中文翻译:

(‐)‐ Ebelactone A的立体选择性全合成
描述了几种治疗上重要的酶(-)-belactone A(1)的β-内酰胺抑制剂的高度立体选择性全合成。该合成的显着特征包括双环对称烯烃的不对称化与布朗的不对称硼氢化,维蒂希烯化,埃文斯烷基化,夏普莱斯不对称环氧化,吉尔曼反应,TEMPO-BAIB介导的1,3-二醇的选择性氧化和亚当的β-内酯化。








































 京公网安备 11010802027423号
京公网安备 11010802027423号