当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing the understanding of hydrogen evolution and oxidation reactions on Pt(111) through ab initio simulation of electrode/electrolyte kinetics
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-04 , DOI: 10.1021/jacs.9b13694
Ling Liu 1 , Yuyang Liu 1 , Chungen Liu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-04 , DOI: 10.1021/jacs.9b13694
Ling Liu 1 , Yuyang Liu 1 , Chungen Liu 1
Affiliation
|
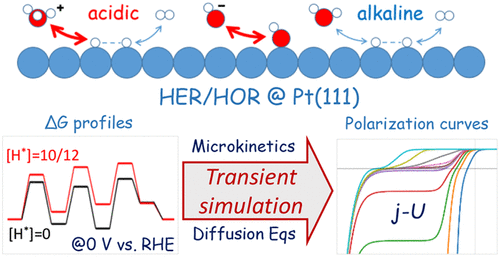
The hydrogen oxidation reaction (HOR) and hydrogen evolution reaction (HER) play an important role in hydrogen-based energy conversion. However, the sluggish kinetics in alkaline media has raised debates on the relevant mechanism, especially on the role of surface hydroxyl (OH*). With the potential-related free energy profiles obtained with density functional theory (DFT) calculations, the full pH range transient kinetics simulation of HER/HOR polarization curves on Pt(111) agrees well with experimental observations. Study on model systems with varying metal-OH* binding energies confirms that the current near the HOR onset potential is contributed from the pathway through OH- other than OH*, suggesting that OH* is unlikely an effective activity descriptor for HOR. Degree of rate control (DRC) analyses reveal that, while acidic current is controlled solely by Tafel step, alkaline current is controlled jointly by Tafel and Volmer steps as the Volmer barrier is considerably increased in alkaline conditions. Finally, based on a model study, we draw up a scheme of reducing the overpotential of alkaline HER/HOR by accelerating the Tafel step.
中文翻译:

通过电极/电解质动力学的从头模拟增强对 Pt(111) 析氢和氧化反应的理解
氢氧化反应(HOR)和析氢反应(HER)在氢基能量转换中起着重要作用。然而,碱性介质中缓慢的动力学引发了对相关机制的争论,尤其是表面羟基 (OH*) 的作用。通过密度泛函理论 (DFT) 计算获得的与势能相关的自由能分布,Pt(111) 上的 HER/HOR 极化曲线的全 pH 范围瞬态动力学模拟与实验观察结果非常吻合。对具有不同金属-OH* 结合能的模型系统的研究证实,HOR 起始电位附近的电流来自通过 OH-而非 OH* 的途径,表明 OH* 不太可能是 HOR 的有效活性描述符。速率控制程度 (DRC) 分析表明,虽然酸性电流仅由 Tafel 步骤控制,但碱性电流由 Tafel 和 Volmer 步骤共同控制,因为 Volmer 势垒在碱性条件下显着增加。最后,基于模型研究,我们制定了通过加速 Tafel 步骤来降低碱性 HER/HOR 过电位的方案。
更新日期:2020-03-04
中文翻译:

通过电极/电解质动力学的从头模拟增强对 Pt(111) 析氢和氧化反应的理解
氢氧化反应(HOR)和析氢反应(HER)在氢基能量转换中起着重要作用。然而,碱性介质中缓慢的动力学引发了对相关机制的争论,尤其是表面羟基 (OH*) 的作用。通过密度泛函理论 (DFT) 计算获得的与势能相关的自由能分布,Pt(111) 上的 HER/HOR 极化曲线的全 pH 范围瞬态动力学模拟与实验观察结果非常吻合。对具有不同金属-OH* 结合能的模型系统的研究证实,HOR 起始电位附近的电流来自通过 OH-而非 OH* 的途径,表明 OH* 不太可能是 HOR 的有效活性描述符。速率控制程度 (DRC) 分析表明,虽然酸性电流仅由 Tafel 步骤控制,但碱性电流由 Tafel 和 Volmer 步骤共同控制,因为 Volmer 势垒在碱性条件下显着增加。最后,基于模型研究,我们制定了通过加速 Tafel 步骤来降低碱性 HER/HOR 过电位的方案。

































 京公网安备 11010802027423号
京公网安备 11010802027423号