当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of Human Chloride Intracellular Channel Protein 1 (CLIC1) with Lipid Bilayers: A Fluorescence Study
Biochemistry ( IF 2.9 ) Pub Date : 2016-06-29 00:00:00 , DOI: 10.1021/acs.biochem.6b00080
Joanna E. Hare 1 , Sophia C. Goodchild 2 , Samuel N. Breit 3 , Paul M. G. Curmi 4 , Louise J. Brown 1
Biochemistry ( IF 2.9 ) Pub Date : 2016-06-29 00:00:00 , DOI: 10.1021/acs.biochem.6b00080
Joanna E. Hare 1 , Sophia C. Goodchild 2 , Samuel N. Breit 3 , Paul M. G. Curmi 4 , Louise J. Brown 1
Affiliation
|
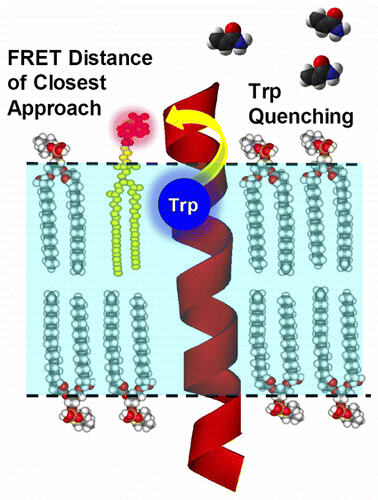
Chloride intracellular channel protein 1 (CLIC1) is very unusual as it adopts a soluble glutathione S-transferase-like canonical fold but can also autoinsert into lipid bilayers to form an ion channel. The conversion between these forms involves a large, but reversible, structural rearrangement of the CLIC1 module. The only identified environmental triggers controlling the metamorphic transition of CLIC1 are pH and oxidation. Until now, there have been no high-resolution structural data available for the CLIC1 integral membrane state, and consequently, a limited understanding of how CLIC1 unfolds and refolds across the bilayer to form a membrane protein with ion channel activity exists. Here we show that fluorescence spectroscopy can be used to establish the interaction and position of CLIC1 in a lipid bilayer. Our method employs a fluorescence energy transfer (FRET) approach between CLIC1 and a dansyl-labeled lipid analogue to probe the CLIC1–lipid interface. Under oxidizing conditions, a strong FRET signal between the single tryptophan residue of CLIC1 (Trp35) and the dansyl-lipid analogue was detected. When considering the proportion of CLIC1 interacting with the lipid bilayer, as estimated by fluorescence quenching experiments, the FRET distance between Trp35 and the dansyl moiety on the membrane surface was determined to be ∼15 Å. This FRET-detected interaction provides direct structural evidence that CLIC1 associates with membranes. The results presented support the current model of an oxidation-driven interaction of CLIC1 with lipid bilayers and also propose a membrane anchoring role for Trp35.
中文翻译:

人氯化物细胞内通道蛋白1(CLIC1)与脂质双层的相互作用:荧光研究。
氯离子细胞内通道蛋白1(CLIC1)非常不寻常,因为它采用了可溶性谷胱甘肽S-转移酶样的典型折叠,但也可以自动插入脂质双层中以形成离子通道。这些形式之间的转换涉及CLIC1模块的大型但可逆的结构重排。唯一确定的控制CLIC1变质转变的环境触发因素是pH和氧化。到目前为止,还没有可用于CLIC1整体膜状态的高分辨率结构数据,因此,对于CLIC1如何在双层中展开和重新折叠以形成具有离子通道活性的膜蛋白的了解有限。在这里我们显示荧光光谱可以用来建立脂质双层中的CLIC1的相互作用和位置。我们的方法在CLIC1和丹磺酰基标记的脂质类似物之间采用荧光能量转移(FRET)方法来探测CLIC1-脂质界面。在氧化条件下,在CLIC1的单个色氨酸残基(Trp35)和丹磺酰脂质类似物之间检测到强FRET信号。当考虑到CLIC1与脂质双层相互作用的比例时,通过荧光猝灭实验估计,在膜表面上Trp35和丹磺酰基部分之间的FRET距离被确定为约15。这种FRET检测到的相互作用提供了CLIC1与膜缔合的直接结构证据。提出的结果支持了CLIC1与脂质双层的氧化驱动相互作用的当前模型,并且还提出了Trp35的膜锚定作用。检测到CLIC1(Trp35)的单个色氨酸残基与丹磺酰脂质类似物之间存在强FRET信号。当考虑到CLIC1与脂质双层相互作用的比例时,通过荧光猝灭实验估计,在膜表面上Trp35和丹磺酰基部分之间的FRET距离被确定为约15。这种FRET检测到的相互作用提供了CLIC1与膜缔合的直接结构证据。提出的结果支持了CLIC1与脂质双层的氧化驱动相互作用的当前模型,并且还提出了Trp35的膜锚定作用。检测到CLIC1(Trp35)的单个色氨酸残基与丹磺酰脂质类似物之间存在强FRET信号。当考虑到CLIC1与脂质双层相互作用的比例时,通过荧光猝灭实验估计,在膜表面上Trp35和丹磺酰基部分之间的FRET距离被确定为约15。这种FRET检测到的相互作用提供了CLIC1与膜缔合的直接结构证据。提出的结果支持了CLIC1与脂质双层的氧化驱动相互作用的当前模型,并且还提出了Trp35的膜锚定作用。通过荧光猝灭实验估计,Trp35和膜表面上的丹磺酰基部分之间的FRET距离约为15。这种FRET检测到的相互作用提供了CLIC1与膜缔合的直接结构证据。提出的结果支持了CLIC1与脂质双层的氧化驱动相互作用的当前模型,并且还提出了Trp35的膜锚定作用。通过荧光猝灭实验估计,Trp35和膜表面上的丹磺酰基部分之间的FRET距离约为15。这种FRET检测到的相互作用提供了CLIC1与膜缔合的直接结构证据。提出的结果支持了CLIC1与脂质双层的氧化驱动相互作用的当前模型,并且还提出了Trp35的膜锚定作用。
更新日期:2016-06-29
中文翻译:

人氯化物细胞内通道蛋白1(CLIC1)与脂质双层的相互作用:荧光研究。
氯离子细胞内通道蛋白1(CLIC1)非常不寻常,因为它采用了可溶性谷胱甘肽S-转移酶样的典型折叠,但也可以自动插入脂质双层中以形成离子通道。这些形式之间的转换涉及CLIC1模块的大型但可逆的结构重排。唯一确定的控制CLIC1变质转变的环境触发因素是pH和氧化。到目前为止,还没有可用于CLIC1整体膜状态的高分辨率结构数据,因此,对于CLIC1如何在双层中展开和重新折叠以形成具有离子通道活性的膜蛋白的了解有限。在这里我们显示荧光光谱可以用来建立脂质双层中的CLIC1的相互作用和位置。我们的方法在CLIC1和丹磺酰基标记的脂质类似物之间采用荧光能量转移(FRET)方法来探测CLIC1-脂质界面。在氧化条件下,在CLIC1的单个色氨酸残基(Trp35)和丹磺酰脂质类似物之间检测到强FRET信号。当考虑到CLIC1与脂质双层相互作用的比例时,通过荧光猝灭实验估计,在膜表面上Trp35和丹磺酰基部分之间的FRET距离被确定为约15。这种FRET检测到的相互作用提供了CLIC1与膜缔合的直接结构证据。提出的结果支持了CLIC1与脂质双层的氧化驱动相互作用的当前模型,并且还提出了Trp35的膜锚定作用。检测到CLIC1(Trp35)的单个色氨酸残基与丹磺酰脂质类似物之间存在强FRET信号。当考虑到CLIC1与脂质双层相互作用的比例时,通过荧光猝灭实验估计,在膜表面上Trp35和丹磺酰基部分之间的FRET距离被确定为约15。这种FRET检测到的相互作用提供了CLIC1与膜缔合的直接结构证据。提出的结果支持了CLIC1与脂质双层的氧化驱动相互作用的当前模型,并且还提出了Trp35的膜锚定作用。检测到CLIC1(Trp35)的单个色氨酸残基与丹磺酰脂质类似物之间存在强FRET信号。当考虑到CLIC1与脂质双层相互作用的比例时,通过荧光猝灭实验估计,在膜表面上Trp35和丹磺酰基部分之间的FRET距离被确定为约15。这种FRET检测到的相互作用提供了CLIC1与膜缔合的直接结构证据。提出的结果支持了CLIC1与脂质双层的氧化驱动相互作用的当前模型,并且还提出了Trp35的膜锚定作用。通过荧光猝灭实验估计,Trp35和膜表面上的丹磺酰基部分之间的FRET距离约为15。这种FRET检测到的相互作用提供了CLIC1与膜缔合的直接结构证据。提出的结果支持了CLIC1与脂质双层的氧化驱动相互作用的当前模型,并且还提出了Trp35的膜锚定作用。通过荧光猝灭实验估计,Trp35和膜表面上的丹磺酰基部分之间的FRET距离约为15。这种FRET检测到的相互作用提供了CLIC1与膜缔合的直接结构证据。提出的结果支持了CLIC1与脂质双层的氧化驱动相互作用的当前模型,并且还提出了Trp35的膜锚定作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号