JACC: Cardiovascular Interventions ( IF 11.7 ) Pub Date : 2020-03-02 , DOI: 10.1016/j.jcin.2019.12.006
Florence Leclercq 1 , Pierre Robert 1 , Mariama Akodad 2 , Jean-Christophe Macia 1 , Thomas Gandet 3 , Delphine Delseny 1 , Marine Chettouh 1 , Laurent Schmutz 4 , Gabriel Robert 5 , Gilles Levy 6 , Frederic Targosz 7 , Eric Maupas 8 , Francois Roubille 2 , Gregory Marin 9 , Nicolas Nagot 9 , Bernard Albat 3 , Benoit Lattuca 4 , Guillaume Cayla 4
|
Objectives
The aim of this study was to evaluate device success of transcatheter aortic valve replacement (TAVR) using new-generation balloon-expandable prostheses with or without balloon aortic valvuloplasty (BAV).
Background
Randomized studies are lacking comparing TAVR without BAV against the conventional technique of TAVR with BAV.
Methods
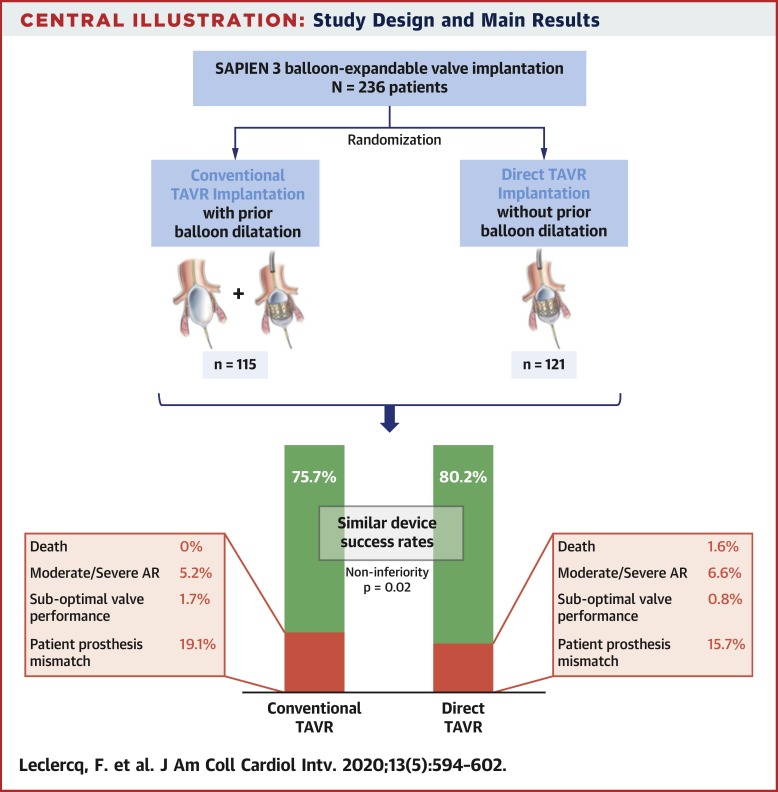
DIRECTAVI (Direct Transcatheter Aortic Valve Implantation) was an open-label noninferiority study that randomized patients undergoing TAVR using the Edwards SAPIEN 3 valve with or without prior balloon valvuloplasty. The primary endpoint was the device success rate according to Valve Academic Research Consortium-2 criteria, which was evaluated using a 7% noninferiority margin. The secondary endpoint included procedural and 30-day adverse events.
Results
Device success was recorded for 184 of 236 included patients (78.0%). The rate of device success in the direct implantation group (n = 97 [80.2%]) was noninferior to that in the BAV group (n = 87 [75.7%]) (mean difference 4.5%; 95% confidence interval: −4.4% to 13.4%; p = 0.02 for noninferiority). No severe prosthesis-patient mismatch or severe aortic regurgitation occurred in any group. In the direct implantation group, 7 patients (5.8%) required BAV to cross the valve. Adverse events were related mainly to pacemaker implantation (20.9% in the BAV group vs. 19.0% in the direct implantation group; p = 0.70). No significant difference was found between the 2 strategies in duration of procedure, contrast volume, radiation exposure, or rate of post-dilatation.
Conclusions
Direct TAVR without prior BAV was noninferior to the conventional strategy using BAV with new-generation balloon-expandable valves, but without procedural simplification. BAV was needed to cross the valve in a few patients, suggesting a need for upstream selection on the basis of patient anatomy. (TAVI Without Balloon Predilatation [of the Aortic Valve] SAPIEN 3 [DIRECTAVI]; NCT02729519)
中文翻译:

先前的球囊瓣膜成形术与直接经导管主动脉瓣置换术:DIRECTAVI试验的结果。
目标
这项研究的目的是评估使用或不使用球囊主动脉瓣膜成形术(BAV)的新一代球囊扩张式假体进行经导管主动脉瓣置换术(TAVR)的设备成功率。
背景
缺乏将没有BAV的TAVR与带有BAV的TAVR的常规技术进行比较的随机研究。
方法
DIRECTAVI(直接经导管主动脉瓣植入术)是一项开放性非劣效性研究,该研究将使用Edwards SAPIEN 3瓣膜进行或不进行球囊瓣膜成形术的TAVR患者随机分组。主要终点是根据Valve学术研究联盟2标准的设备成功率,该标准使用7%的非劣质性进行评估。次要终点包括手术和30天不良事件。
结果
记录了236名患者中的184名患者的设备成功(78.0%)。直接植入组的器械成功率(n = 97 [80.2%])不逊于BAV组的器械成功率(n = 87 [75.7%])(平均差异4.5%; 95%的置信区间:-4.4%至13.4%;非劣效性p = 0.02)。任何一组均没有发生严重的假体-患者失配或严重的主动脉瓣关闭不全。在直接植入组中,有7名患者(5.8%)需要BAV才能通过瓣膜。不良事件主要与起搏器植入有关(BAV组为20.9%,而直接植入组为19.0%; p = 0.70)。两种方法在手术时间,造影剂体积,放射线照射或扩张后率方面均未发现明显差异。
结论
不使用先前的BAV的直接TAVR与使用带有新一代球囊可扩张瓣膜的BAV的传统策略相比并不逊色,但没有程序上的简化。少数患者需要BAV穿过瓣膜,这表明需要根据患者的解剖结构进行上游选择。(无主动脉瓣球囊扩张术的TAVI SAPIEN 3 [DIRECTAVI]; NCT02729519)































 京公网安备 11010802027423号
京公网安备 11010802027423号