Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimal Mutant Model of Human S100A3 Protein Citrullinated at Arg51 by Peptidylarginine Deiminase Type III and Its Solution Structural Properties
ACS Omega ( IF 3.7 ) Pub Date : 2020-02-18 , DOI: 10.1021/acsomega.9b03618 Kenji Ite 1, 2 , Kento Yonezawa 3 , Kenichi Kitanishi 1, 2 , Nobutaka Shimizu 3 , Masaki Unno 1, 2
ACS Omega ( IF 3.7 ) Pub Date : 2020-02-18 , DOI: 10.1021/acsomega.9b03618 Kenji Ite 1, 2 , Kento Yonezawa 3 , Kenichi Kitanishi 1, 2 , Nobutaka Shimizu 3 , Masaki Unno 1, 2
Affiliation
|
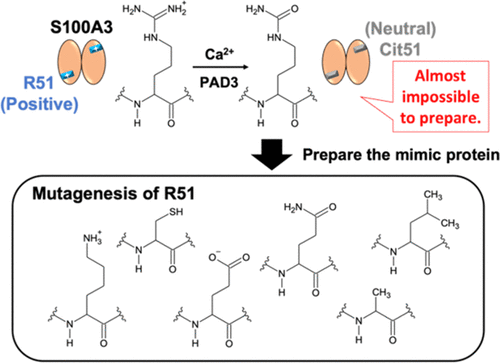
S100A3 protein, a member of the EF-hand-type Ca2+-binding S100 protein family, undergoes a Ca2+-/Zn2+-induced structural change to a tetrameric state upon specific citrullination of R51 in human hair cuticular cells. To elucidate the underlying mechanism, we prepared recombinant mutant S100A3 proteins, including R51A, R51C, R51E, R51K, and R51Q, as potential models of post-translationally modified S100A3 and evaluated their biophysical and biochemical properties relative to wild-type (WT) S100A3 and WT citrullinated in vitro. Size exclusion chromatography (SEC) showed that R51Q formed a tetramer in the presence of Ca2+, while Ca2+ titration monitored by Trp fluorescence indicated that R51Q had Ca2+-binding properties similar to those of citrullinated S1003A. We therefore concluded that R51Q is the optimal mutant model of post-translationally modified S100A3. We compared the solution structure of WT S100A3 and the R51Q mutant in the absence and presence of Ca2+ and Zn2+ by SEC-small-angle X-ray scattering. The radius of gyration of R51Q in the metal-free state was almost the same as that of WT; however, it increased by ∼1.5-fold in the presence of Ca2+/Zn2+, indicating a large expansion in molecular size. By contrast, addition of Ca2+/Zn2+ to WT led to nonspecific aggregation in SEC analysis and dynamic light scattering, suggesting that citrullination of S100A3 is essential for stabilization of the Ca2+-/Zn2+-bound state. These findings will lead to the further development of structural analyses for the Ca2+-/Zn2+-bound S100A3.
中文翻译:

肽基精氨酸脱亚胺酶Ⅲ型人S100A3蛋白Arg51瓜氨酸化的最佳突变模型及其溶液结构性质
S100A3 蛋白是 EF 手型 Ca 2+结合 S100 蛋白家族的成员,在人毛角质层细胞中 R51 特异性瓜氨酸化后,经历 Ca 2+ -/Zn 2+诱导的结构变化至四聚体状态。为了阐明其潜在机制,我们制备了重组突变S100A3蛋白,包括R51A、R51C、R51E、R51K和R51Q,作为翻译后修饰S100A3的潜在模型,并评估了它们相对于野生型(WT)S100A3的生物物理和生化特性。和WT体外瓜氨酸化。尺寸排阻色谱(SEC)显示R51Q在Ca 2+存在下形成四聚体,而通过Trp荧光监测的Ca 2+滴定表明R51Q具有与瓜氨酸化S1003A相似的Ca 2+结合特性。因此我们得出结论,R51Q是翻译后修饰的S100A3的最佳突变模型。我们通过SEC小角X射线散射比较了WT S100A3和R51Q突变体在Ca 2+和Zn 2+不存在和存在下的溶液结构。 R51Q在无金属状态下的回转半径与WT几乎相同;然而,在Ca 2+ /Zn 2+存在下,它增加了约1.5倍,表明分子尺寸大幅膨胀。相比之下,向WT添加Ca 2+ /Zn 2+导致SEC分析和动态光散射中的非特异性聚集,这表明S100A3的瓜氨酸化对于Ca 2+ -/Zn 2+ -结合状态的稳定至关重要。 这些发现将促进Ca 2+ -/Zn 2+结合的S100A3 结构分析的进一步发展。
更新日期:2020-03-03
中文翻译:

肽基精氨酸脱亚胺酶Ⅲ型人S100A3蛋白Arg51瓜氨酸化的最佳突变模型及其溶液结构性质
S100A3 蛋白是 EF 手型 Ca 2+结合 S100 蛋白家族的成员,在人毛角质层细胞中 R51 特异性瓜氨酸化后,经历 Ca 2+ -/Zn 2+诱导的结构变化至四聚体状态。为了阐明其潜在机制,我们制备了重组突变S100A3蛋白,包括R51A、R51C、R51E、R51K和R51Q,作为翻译后修饰S100A3的潜在模型,并评估了它们相对于野生型(WT)S100A3的生物物理和生化特性。和WT体外瓜氨酸化。尺寸排阻色谱(SEC)显示R51Q在Ca 2+存在下形成四聚体,而通过Trp荧光监测的Ca 2+滴定表明R51Q具有与瓜氨酸化S1003A相似的Ca 2+结合特性。因此我们得出结论,R51Q是翻译后修饰的S100A3的最佳突变模型。我们通过SEC小角X射线散射比较了WT S100A3和R51Q突变体在Ca 2+和Zn 2+不存在和存在下的溶液结构。 R51Q在无金属状态下的回转半径与WT几乎相同;然而,在Ca 2+ /Zn 2+存在下,它增加了约1.5倍,表明分子尺寸大幅膨胀。相比之下,向WT添加Ca 2+ /Zn 2+导致SEC分析和动态光散射中的非特异性聚集,这表明S100A3的瓜氨酸化对于Ca 2+ -/Zn 2+ -结合状态的稳定至关重要。 这些发现将促进Ca 2+ -/Zn 2+结合的S100A3 结构分析的进一步发展。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号