当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Screening of Imidazole Ionic Liquids for Separating the Acetone–n-Hexane Azeotrope by COSMO-SAC Simulations and Experimental Verification
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-03-10 , DOI: 10.1021/acssuschemeng.9b07358 Yinglong Wang 1 , Xiao Yang 1 , Wenting Bai 1 , Jie Zhang 1 , Xinran Zhou 1 , Xiaokai Guo 1 , Jincheng Peng 1 , Jianguang Qi 1 , Zhaoyou Zhu 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-03-10 , DOI: 10.1021/acssuschemeng.9b07358 Yinglong Wang 1 , Xiao Yang 1 , Wenting Bai 1 , Jie Zhang 1 , Xinran Zhou 1 , Xiaokai Guo 1 , Jincheng Peng 1 , Jianguang Qi 1 , Zhaoyou Zhu 1
Affiliation
|
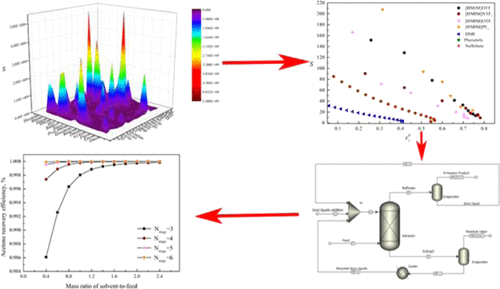
Ionic liquids have received wide attention as green recyclable extractants. In view of their broad applicability, in the current work, ionic liquids were explored for their ability to extract acetone from n-hexane. Based on the conductor-like screening model for the segment activity coefficient, the surface charge densities of 15 kinds of cations, 19 kinds of anions, acetone, and n-hexane were calculated via quantum chemistry approaches. The infinite dilution activity coefficients of the ionic liquid–acetone–n-hexane system were calculated, and the distribution coefficient and selectivity of the system were determined. The effective extractants [1-butyl-3-methylimidazolium trifluoromethanesulfonate, 1-hexyl-3-methylimidazolium bis(trifluoromethanesulfonyl), 1-hexyl-3-methylimidazolium trifluoromethanesulfonate, and 1-hexyl-3-methylimidazolium hexafluorophosphate] were selected. The experimental data of the liquid–liquid equilibrium were correlated by the NRTL and UNIQUAC models. The regression of the experimental data gave the binary interaction parameters of the two models. In addition, a process of extracting and separating the n-hexane–acetone azeotrope system using Aspen Plus 8.4 was proposed and the parameters were analyzed. Based on the NRTL model, a continuous extraction process using ionic liquids as the extractant was simulated. Ionic liquids are a promising solvent for the extraction and separation of acetone and n-hexane.
中文翻译:

咪唑离子液体的筛选用于分离丙酮Ñ -己烷共沸物通过COSMO-SAC模拟和实验验证
离子液体作为绿色可回收萃取剂已受到广泛关注。鉴于它们的广泛的适用性的,在目前的工作中,离子液体进行了探索它们从能力提取丙酮Ñ正己烷。基于所述导体状筛选模型为段活度系数,15种阳离子的19种阴离子,丙酮的表面电荷密度,以及Ñ己烷计算通过量子化学方法。离子液体–丙酮– n的无限稀释活度系数计算了正己烷体系,并确定了体系的分布系数和选择性。选择有效的萃取剂[1-丁基-3-甲基咪唑三氟甲磺酸盐,1-己基-3-甲基咪唑双(三氟甲磺酰基),1-己基-3-甲基咪唑三氟甲磺酸盐和1-己基-3-甲基咪唑六氟磷酸盐。液-液平衡的实验数据通过NRTL和UNIQUAC模型进行关联。实验数据的回归给出了两个模型的二元相互作用参数。另外,提取和分离n的过程提出了使用Aspen Plus 8.4的正己烷-丙酮共沸体系,并对参数进行了分析。基于NRTL模型,模拟了使用离子液体作为萃取剂的连续萃取过程。离子液体是在萃取和丙酮的分离和有希望的溶剂Ñ正己烷。
更新日期:2020-03-10
中文翻译:

咪唑离子液体的筛选用于分离丙酮Ñ -己烷共沸物通过COSMO-SAC模拟和实验验证
离子液体作为绿色可回收萃取剂已受到广泛关注。鉴于它们的广泛的适用性的,在目前的工作中,离子液体进行了探索它们从能力提取丙酮Ñ正己烷。基于所述导体状筛选模型为段活度系数,15种阳离子的19种阴离子,丙酮的表面电荷密度,以及Ñ己烷计算通过量子化学方法。离子液体–丙酮– n的无限稀释活度系数计算了正己烷体系,并确定了体系的分布系数和选择性。选择有效的萃取剂[1-丁基-3-甲基咪唑三氟甲磺酸盐,1-己基-3-甲基咪唑双(三氟甲磺酰基),1-己基-3-甲基咪唑三氟甲磺酸盐和1-己基-3-甲基咪唑六氟磷酸盐。液-液平衡的实验数据通过NRTL和UNIQUAC模型进行关联。实验数据的回归给出了两个模型的二元相互作用参数。另外,提取和分离n的过程提出了使用Aspen Plus 8.4的正己烷-丙酮共沸体系,并对参数进行了分析。基于NRTL模型,模拟了使用离子液体作为萃取剂的连续萃取过程。离子液体是在萃取和丙酮的分离和有希望的溶剂Ñ正己烷。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号