当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogen Atom Transfer Oxidation by a Gold-Hydroxide Complex.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-03-03 , DOI: 10.1021/acs.inorgchem.9b03225 Marta Lovisari 1 , Aidan R McDonald 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-03-03 , DOI: 10.1021/acs.inorgchem.9b03225 Marta Lovisari 1 , Aidan R McDonald 1
Affiliation
|
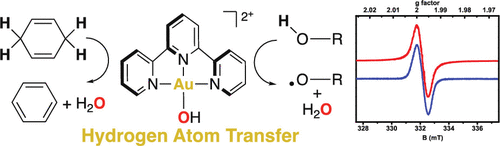
AuIII-oxygen adducts have been implicated as intermediates in homogeneous and heterogeneous Au oxidation catalysis, but their reactivity is under-explored. Complex 1, ([AuIII(OH)(terpy)](ClO4)2, (terpy = 2,2':6',2-terpyridine), readily oxidized substrates bearing C-H and O-H bonds. Kinetic analysis revealed that the oxidation occurred through a hydrogen atom transfer (HAT) mechanism. Stable radicals were detected and quantified as products of almost quantitative HAT oxidation of alcohols by 1. Our findings highlight the possible role of AuIII-oxygen adducts in oxidation catalysis and the capability of late transition metal-oxygen adducts to perform proton coupled electron transfer.
中文翻译:

氢原子转移氧化金氢氧化物。
AuIII-氧加合物已被暗示为均相和非均相Au氧化催化的中间体,但其反应性尚未得到充分研究。配合物1([AuIII(OH)(terpy)](ClO4)2,(terpy = 2,2':6',2-terridine),易于氧化的带有CH和OH键的底物。动力学分析表明发生了氧化通过氢原子转移(HAT)机理检测出稳定的自由基,并将其量化为几乎定量的HAT酒精氧化产物。我们的发现突显了AuIII-氧加合物在氧化催化中的可能作用以及后期过渡金属的能力。氧加合物执行质子耦合电子转移。
更新日期:2020-03-03
中文翻译:

氢原子转移氧化金氢氧化物。
AuIII-氧加合物已被暗示为均相和非均相Au氧化催化的中间体,但其反应性尚未得到充分研究。配合物1([AuIII(OH)(terpy)](ClO4)2,(terpy = 2,2':6',2-terridine),易于氧化的带有CH和OH键的底物。动力学分析表明发生了氧化通过氢原子转移(HAT)机理检测出稳定的自由基,并将其量化为几乎定量的HAT酒精氧化产物。我们的发现突显了AuIII-氧加合物在氧化催化中的可能作用以及后期过渡金属的能力。氧加合物执行质子耦合电子转移。































 京公网安备 11010802027423号
京公网安备 11010802027423号