当前位置:
X-MOL 学术
›
ACS Appl. Nano Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stable CsPbBr3:Sn@SiO2 and Cs4PbBr6:Sn@SiO2 Core–Shell Quantum Dots with Tunable Color Emission for Light-Emitting Diodes
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2020-03-02 , DOI: 10.1021/acsanm.0c00299 Bo Wang 1 , Shan Zhang 1 , Bin Liu 1 , Jinkai Li 1 , Bingqiang Cao 1 , Zongming Liu 1
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2020-03-02 , DOI: 10.1021/acsanm.0c00299 Bo Wang 1 , Shan Zhang 1 , Bin Liu 1 , Jinkai Li 1 , Bingqiang Cao 1 , Zongming Liu 1
Affiliation
|
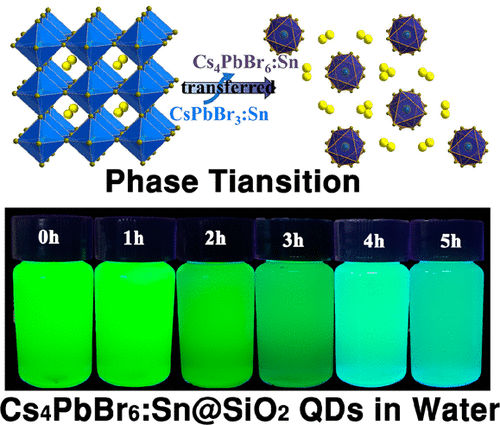
CsPbBr3 perovskite quantum dots (QDs) have attracted great attention due to their different photoluminescent and electronic properties. However, the toxicity and low stability hinder their practical application. Here, low-toxicity Sn-substituted cesium lead bromide perovskite QDs were synthesized via the room temperature crystallization method. A phase transition of the perovskite quantum dots with the concentration of Sn increasing was found: the crystal phase of CsPbBr3:Sn perovskite QDs starts to be transformed into Cs4PbBr6:Sn perovskite QDs when the SnBr2 precursor exceeds 30 at. %. In this process, the controlled-color emission of perovskite QDs from green to blue can be realized. To improve the stability of quantum dots, CsPbBr3:Sn@SiO2 and Cs4PbBr6:Sn@SiO2 were synthesized via a simple one-step synthesis with hydrolysis of tetramethoxysilane in toluene solution containing perovskite QDs. The results show that CsPbBr3:Sn@SiO2 and Cs4PbBr6:Sn@SiO2 exhibited higher water stability and water solubility than pure QDs. In a mixed solution of toluene and water, the PL intensity of the CsPbBr3:Sn@SiO2 retained 46.7% and the Cs4PbBr6:Sn@SiO2 was 59.6% even after 24 h of reaction compared with the initial intensities. These quantum dots with good stability and luminescent property developed in this work are expected to be widely used in light-emitting diodes.
中文翻译:

稳定的CsPbBr 3:Sn @ SiO 2和Cs 4 PbBr 6:Sn @ SiO 2核-壳量子点,可调节发光二极管的颜色
CsPbBr 3钙钛矿量子点(QDs)由于其不同的光致发光和电子特性而备受关注。但是,毒性和低稳定性阻碍了它们的实际应用。在此,通过室温结晶法合成了低毒的Sn-取代的溴化铯铅钙钛矿QD。发现随着Sn浓度的增加,钙钛矿量子点的相变:当SnBr 2时,CsPbBr 3:Sn钙钛矿QDs的晶体相开始转变为Cs 4 PbBr 6:Sn钙钛矿QDs。前体超过30 at。%。在此过程中,可以实现钙钛矿量子点从绿色到蓝色的受控颜色发射。为了提高量子点的稳定性,在含钙钛矿QDs的甲苯溶液中通过四甲氧基硅烷的水解,通过简单的一步合成来合成CsPbBr 3:Sn @ SiO 2和Cs 4 PbBr 6:Sn @ SiO 2。结果表明,CsPbBr 3:Sn @ SiO 2和Cs 4 PbBr 6:Sn @ SiO 2比纯QDs具有更高的水稳定性和水溶性。在甲苯和水的混合溶液中,CsPbBr 3:Sn @ SiO的PL强度与初始强度相比,即使在反应24小时后,2仍保留46.7%,Cs 4 PbBr 6:Sn @ SiO 2仍为59.6%。期望在这项工作中开发的具有良好稳定性和发光特性的这些量子点将广泛用于发光二极管中。
更新日期:2020-03-02
中文翻译:

稳定的CsPbBr 3:Sn @ SiO 2和Cs 4 PbBr 6:Sn @ SiO 2核-壳量子点,可调节发光二极管的颜色
CsPbBr 3钙钛矿量子点(QDs)由于其不同的光致发光和电子特性而备受关注。但是,毒性和低稳定性阻碍了它们的实际应用。在此,通过室温结晶法合成了低毒的Sn-取代的溴化铯铅钙钛矿QD。发现随着Sn浓度的增加,钙钛矿量子点的相变:当SnBr 2时,CsPbBr 3:Sn钙钛矿QDs的晶体相开始转变为Cs 4 PbBr 6:Sn钙钛矿QDs。前体超过30 at。%。在此过程中,可以实现钙钛矿量子点从绿色到蓝色的受控颜色发射。为了提高量子点的稳定性,在含钙钛矿QDs的甲苯溶液中通过四甲氧基硅烷的水解,通过简单的一步合成来合成CsPbBr 3:Sn @ SiO 2和Cs 4 PbBr 6:Sn @ SiO 2。结果表明,CsPbBr 3:Sn @ SiO 2和Cs 4 PbBr 6:Sn @ SiO 2比纯QDs具有更高的水稳定性和水溶性。在甲苯和水的混合溶液中,CsPbBr 3:Sn @ SiO的PL强度与初始强度相比,即使在反应24小时后,2仍保留46.7%,Cs 4 PbBr 6:Sn @ SiO 2仍为59.6%。期望在这项工作中开发的具有良好稳定性和发光特性的这些量子点将广泛用于发光二极管中。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号