当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cooperative Pd(0)/Rh(II) Dual Catalysis: Interceptive Capturing of π-Allyl Pd(II) Complexes with α-Imino Rh(II) Carbenoids
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-06-28 00:00:00 , DOI: 10.1021/acscatal.6b01110 Zi-Sheng Chen 1, 2 , Liang-Zhu Huang 1, 2 , Hyun Ji Jeon 2 , Zi Xuan 2 , Sang-gi Lee 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-06-28 00:00:00 , DOI: 10.1021/acscatal.6b01110 Zi-Sheng Chen 1, 2 , Liang-Zhu Huang 1, 2 , Hyun Ji Jeon 2 , Zi Xuan 2 , Sang-gi Lee 2
Affiliation
|
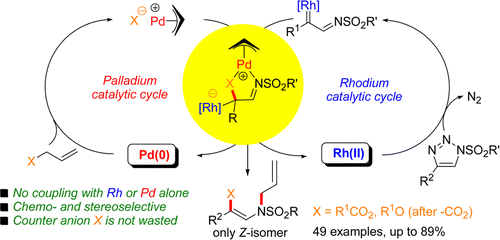
Extensive effort has been expended to utilize π-allyl palladium complexes as electrophilic allyl donor intermediates in cooperative dual catalysis, but their counteranions such as carboxylates and alkoxides are almost always discarded as waste. We have developed a cooperative Pd(0)/Rh(II) dual-catalysis system that utilizes both electrophilic allyl and nucleophilic counteranion functionalities inherent in the starting allylic substrates. In this cooperative catalysis, redox-compatible Pd(0) and Rh(II) catalysts selectively activate allylic substrates and N-sulfonyl-1,2,3-triazoles to generate π-allyl Pd(II) complexes and 1,3-ambivalent equivalent α-imino Rh(II) carbenoid intermediates, respectively. The counteranion of the π-allyl Pd(II) complex acts as a nucleophile transferring to the electrophilic carbenic carbon to form Pd/Rh-associated zwitterionic intermediates, in which the cationic palladium species may coordinate with both counteranion and imine nitrogen in the same plane, establishing the Z geometry of the products.
中文翻译:

协同Pd(0)/ Rh(II)双重催化:与α-氨基Rh(II)类胡萝卜素的π-烯丙基Pd(II)配合物的拦截捕获
在协同双重催化中已经花费大量努力来利用π-烯丙基钯配合物作为亲电子烯丙基供体中间体,但是它们的抗衡阴离子例如羧酸根和醇根几乎总是作为废物丢弃。我们已经开发了一种合作的Pd(0)/ Rh(II)双催化系统,该系统利用了起始烯丙基底物中固有的亲电烯丙基和亲核抗衡阴离子功能。在这种协同催化中,氧化还原兼容的Pd(0)和Rh(II)催化剂选择性活化烯丙基底物和N-磺酰基-1,2,3-三唑分别生成π-烯丙基Pd(II)配合物和1,3-二价等效α-亚氨基Rh(II)类胡萝卜素中间体。π-烯丙基Pd(II)络合物的抗衡阴离子充当亲核试剂转移到亲电碳碳上,形成Pd / Rh相关的两性离子中间体,其中阳离子钯物质可与抗衡阴离子和亚胺氮在同一平面上同时存在,确定产品的Z几何形状。
更新日期:2016-06-28
中文翻译:

协同Pd(0)/ Rh(II)双重催化:与α-氨基Rh(II)类胡萝卜素的π-烯丙基Pd(II)配合物的拦截捕获
在协同双重催化中已经花费大量努力来利用π-烯丙基钯配合物作为亲电子烯丙基供体中间体,但是它们的抗衡阴离子例如羧酸根和醇根几乎总是作为废物丢弃。我们已经开发了一种合作的Pd(0)/ Rh(II)双催化系统,该系统利用了起始烯丙基底物中固有的亲电烯丙基和亲核抗衡阴离子功能。在这种协同催化中,氧化还原兼容的Pd(0)和Rh(II)催化剂选择性活化烯丙基底物和N-磺酰基-1,2,3-三唑分别生成π-烯丙基Pd(II)配合物和1,3-二价等效α-亚氨基Rh(II)类胡萝卜素中间体。π-烯丙基Pd(II)络合物的抗衡阴离子充当亲核试剂转移到亲电碳碳上,形成Pd / Rh相关的两性离子中间体,其中阳离子钯物质可与抗衡阴离子和亚胺氮在同一平面上同时存在,确定产品的Z几何形状。















































 京公网安备 11010802027423号
京公网安备 11010802027423号