当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced nitrate-to-ammonia activity on copper-nickel alloys via tuning of intermediate adsorption
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-02 , DOI: 10.1021/jacs.9b13347 Yuhang Wang 1 , Aoni Xu 1 , Ziyun Wang 1 , Linsong Huang 2 , Jun Li 1, 3 , Fengwang Li 1 , Joshua Wicks 1 , Mingchuan Luo 1 , Dae-Hyun Nam 1 , Chih-Shan Tan 1 , Yu Ding 2 , Jiawen Wu 2 , Yanwei Lum 1 , Cao-Thang Dinh 1 , David Sinton 3 , Gengfeng Zheng 2 , Edward H Sargent 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-02 , DOI: 10.1021/jacs.9b13347 Yuhang Wang 1 , Aoni Xu 1 , Ziyun Wang 1 , Linsong Huang 2 , Jun Li 1, 3 , Fengwang Li 1 , Joshua Wicks 1 , Mingchuan Luo 1 , Dae-Hyun Nam 1 , Chih-Shan Tan 1 , Yu Ding 2 , Jiawen Wu 2 , Yanwei Lum 1 , Cao-Thang Dinh 1 , David Sinton 3 , Gengfeng Zheng 2 , Edward H Sargent 1
Affiliation
|
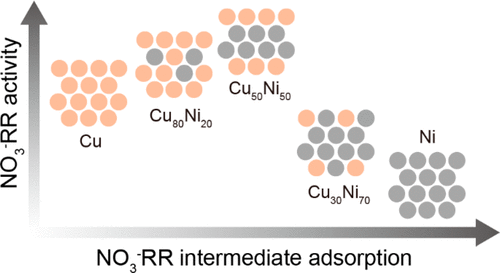
Electrochemical conversion of NO3- into ammonia (NH3) recycles nitrogen and offers a route to NH3 production that is more valuable than dinitrogen gas. However, today's development of NO3- electroreduction remains hindered by the lack of a mechanistic picture of how catalyst structure may be tuned to enhance catalytic activity. Here we demonstrate enhanced nitrate reduction reaction (NO3-RR) performance on Cu50Ni50 alloy catalysts, including a 0.12 V upshift in the half-wave potential and a 6-fold increase in activity compared to pure Cu at 0 V vs. reversible hydrogen electrode (RHE). Ni alloying enables tuning of the Cu d-band center and modulates the adsorption energies of intermediates such as *NO3, *NO2, and *NH2. Using density functional theory (DFT) calculations, we identify a NO3-RR-to-NH3 pathway and offer an adsorption energy-activity relationship for the CuNi alloy system. This correlation between catalyst electronic structure and NO3-RR activity offers a design platform for further development of NO3-RR catalysts.
中文翻译:

通过调节中间吸附增强铜镍合金硝酸盐到氨的活性
将 NO3- 电化学转化为氨 (NH3) 可循环使用氮气,并提供一条比氮气更有价值的 NH3 生产途径。然而,由于缺乏如何调整催化剂结构以提高催化活性的机理图,今天 NO3 电还原的发展仍然受到阻碍。在这里,我们证明了 Cu50Ni50 合金催化剂的硝酸盐还原反应 (NO3-RR) 性能增强,包括半波电位上移 0.12 V,与 0 V 下的纯铜相比,活性增加了 6 倍与可逆氢电极( RHE)。Ni 合金化能够调节 Cu d 带中心并调节中间体的吸附能,例如 *NO3、*NO2 和 *NH2。使用密度泛函理论 (DFT) 计算,我们确定了 NO3-RR 到 NH3 的途径,并提供了 CuNi 合金系统的吸附能量-活性关系。催化剂电子结构与 NO3-RR 活性之间的这种相关性为进一步开发 NO3-RR 催化剂提供了设计平台。
更新日期:2020-03-02
中文翻译:

通过调节中间吸附增强铜镍合金硝酸盐到氨的活性
将 NO3- 电化学转化为氨 (NH3) 可循环使用氮气,并提供一条比氮气更有价值的 NH3 生产途径。然而,由于缺乏如何调整催化剂结构以提高催化活性的机理图,今天 NO3 电还原的发展仍然受到阻碍。在这里,我们证明了 Cu50Ni50 合金催化剂的硝酸盐还原反应 (NO3-RR) 性能增强,包括半波电位上移 0.12 V,与 0 V 下的纯铜相比,活性增加了 6 倍与可逆氢电极( RHE)。Ni 合金化能够调节 Cu d 带中心并调节中间体的吸附能,例如 *NO3、*NO2 和 *NH2。使用密度泛函理论 (DFT) 计算,我们确定了 NO3-RR 到 NH3 的途径,并提供了 CuNi 合金系统的吸附能量-活性关系。催化剂电子结构与 NO3-RR 活性之间的这种相关性为进一步开发 NO3-RR 催化剂提供了设计平台。































 京公网安备 11010802027423号
京公网安备 11010802027423号