当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemotaxis-driven delivery of nano-pathogenoids for complete eradication of tumors post-phototherapy.
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-28 , DOI: 10.1038/s41467-020-14963-0 Min Li 1, 2, 3 , Shuya Li 1 , Han Zhou 1 , Xinfeng Tang 1 , Yi Wu 1 , Wei Jiang 1 , Zhigang Tian 1 , Xuechang Zhou 3 , Xianzhu Yang 4 , Yucai Wang 1, 2
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-28 , DOI: 10.1038/s41467-020-14963-0 Min Li 1, 2, 3 , Shuya Li 1 , Han Zhou 1 , Xinfeng Tang 1 , Yi Wu 1 , Wei Jiang 1 , Zhigang Tian 1 , Xuechang Zhou 3 , Xianzhu Yang 4 , Yucai Wang 1, 2
Affiliation
|
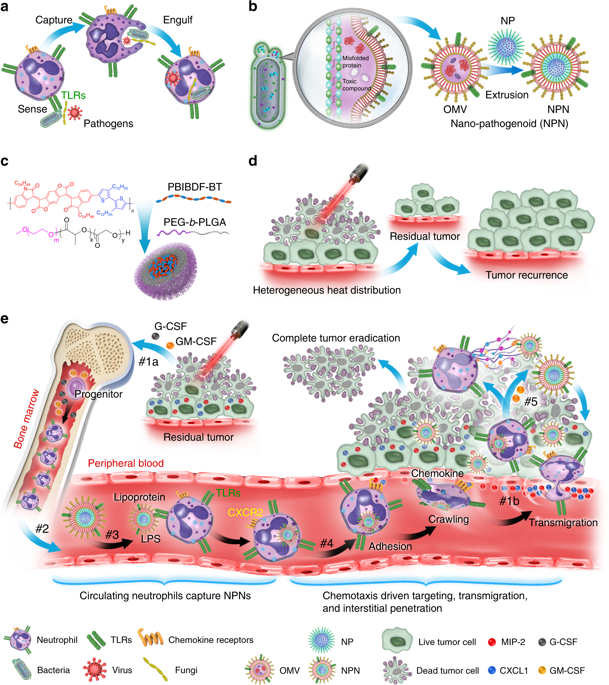
The efficacy of nano-mediated drug delivery has been impeded by multiple biological barriers such as the mononuclear phagocyte system (MPS), as well as vascular and interstitial barriers. To overcome the abovementioned obstacles, we report a nano-pathogenoid (NPN) system that can in situ hitchhike circulating neutrophils and supplement photothermal therapy (PTT). Cloaked with bacteria-secreted outer membrane vesicles inheriting pathogen-associated molecular patterns of native bacteria, NPNs are effectively recognized and internalized by neutrophils. The neutrophils migrate towards inflamed tumors, extravasate across the blood vessels, and penetrate through the tumors. Then NPNs are rapidly released from neutrophils in response to inflammatory stimuli and subsequently taken up by tumor cells to exert anticancer effects. Strikingly, due to the excellent targeting efficacy, cisplatin-loaded NPNs combined with PTT completely eradicate tumors in all treated mice. Such a nano-platform represents an efficient and generalizable strategy towards in situ cell hitchhiking as well as enhanced tumor targeted delivery.
中文翻译:

趋化性驱动的纳米病原菌的递送,用于彻底根除光疗后的肿瘤。
纳米介导的药物递送的功效已受到多种生物屏障(例如单核吞噬细胞系统(MPS))以及血管和间质屏障的阻碍。为了克服上述障碍,我们报告了一种纳米病原体(NPN)系统,该系统可以原位搭便车循环中性粒细胞并补充光热疗法(PTT)。NPNs被细菌分泌的外膜囊泡所掩盖,该囊泡继承了天然细菌的病原体相关分子模式,NPNs被中性粒细胞有效地识别和内化。中性粒细胞向发炎的肿瘤迁移,在血管中扩散,并穿透肿瘤。然后,NPNs响应炎症刺激而从嗜中性粒细胞中迅速释放出来,随后被肿瘤细胞吸收以发挥抗癌作用。惊人地 由于出色的靶向功效,顺铂加载的NPNs与PTT结合可完全根除所有治疗小鼠的肿瘤。这样的纳米平台代表了一种有效且可推广的策略,用于原位细胞搭便车以及增强的肿瘤靶向递送。
更新日期:2020-02-28
中文翻译:

趋化性驱动的纳米病原菌的递送,用于彻底根除光疗后的肿瘤。
纳米介导的药物递送的功效已受到多种生物屏障(例如单核吞噬细胞系统(MPS))以及血管和间质屏障的阻碍。为了克服上述障碍,我们报告了一种纳米病原体(NPN)系统,该系统可以原位搭便车循环中性粒细胞并补充光热疗法(PTT)。NPNs被细菌分泌的外膜囊泡所掩盖,该囊泡继承了天然细菌的病原体相关分子模式,NPNs被中性粒细胞有效地识别和内化。中性粒细胞向发炎的肿瘤迁移,在血管中扩散,并穿透肿瘤。然后,NPNs响应炎症刺激而从嗜中性粒细胞中迅速释放出来,随后被肿瘤细胞吸收以发挥抗癌作用。惊人地 由于出色的靶向功效,顺铂加载的NPNs与PTT结合可完全根除所有治疗小鼠的肿瘤。这样的纳米平台代表了一种有效且可推广的策略,用于原位细胞搭便车以及增强的肿瘤靶向递送。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号