当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyclometalated Pt Complexes of CNC Pincer Ligands: Luminescence and Cytotoxic Evaluation
Organometallics ( IF 2.5 ) Pub Date : 2020-02-27 , DOI: 10.1021/acs.organomet.0c00015
Simon Garbe 1 , Maren Krause 1 , Annika Klimpel 2 , Ines Neundorf 2 , Petra Lippmann 3 , Ingo Ott 3 , Dana Brünink 4 , Cristian A. Strassert 5 , Nikos L. Doltsinis 4 , Axel Klein 1
Organometallics ( IF 2.5 ) Pub Date : 2020-02-27 , DOI: 10.1021/acs.organomet.0c00015
Simon Garbe 1 , Maren Krause 1 , Annika Klimpel 2 , Ines Neundorf 2 , Petra Lippmann 3 , Ingo Ott 3 , Dana Brünink 4 , Cristian A. Strassert 5 , Nikos L. Doltsinis 4 , Axel Klein 1
Affiliation
|
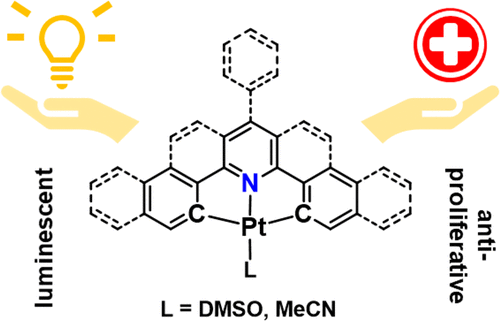
In the framework of our attempts to develop cyclometalated Pt(II) complexes toward bifunctional targeting inhibitors or agents for photodynamic therapy, diagnostics, and bioimaging, a series of bis-cyclometalated Pt(II) complexes [Pt(CNC)(L)] (L = DMSO, MeCN) containing various (CNC)2– ligands based on 2,6-diphenylpyridine were synthesized and characterized analytically and spectroscopically, focusing on their electrochemical, luminescence, and antiproliferative properties. Electrochemical experiments and UV–vis absorption spectroscopy suggest ligand-centered LUMOs and metal-centered HOMOs in line with DFT calculations. Extension of the ancillary phenyl to naphthyl cores and a central 4-phenylpyridine group instead of pyridine results in bathochromic shifts of the long-wavelength absorption bands ranging from 420 to 440 nm, with the latter shift being more pronounced. The complexes of the fused CNC heterocyclic systems dba (H2dba = dibenzo[c,h]acridine), db(ph)a (H2db(ph)a = 7-phenyldibenzo[c,h]acridine), and bzqph (HbzqphH = 2-phenylbenzo[h]quinoline) absorb far more red-shifted in the range 500–530 nm. All complexes show reversible first electrochemical reductions and irreversible oxidations with an electrochemical gap of about 3 V, roughly in line with the absorption energies. While the 2,6-diphenylpyridine complexes [Pt(CNC)(DMSO)] show no luminescence at ambient temperature in solution, the fused dba, db(ph)a, and bzqph derivatives are efficient triplet emitters at ambient temperature with emission wavelengths in the region 575–600 nm and quantum yields ranging from 7 to 23%. Vibrationally resolved emission spectra calculated in the framework of DFT faithfully reproduce the experimental data. TD-DFT calculations at the excited-state T1 geometry reveal intraligand π–π*/MLCT character of the emission for all three investigated complexes. Antiproliferative tests on selected complexes gave very different toxicities, ranging from lower than 1 μM to virtually nontoxic. The data allowed drawing some structure–activity relationships (SAR), even though variations in solubility could also significantly account for the different toxicities.
中文翻译:

CNC钳配体的环金属化Pt配合物:发光和细胞毒性评估
在我们尝试开发用于双功能靶向抑制剂或光动力疗法,诊断学和生物成像试剂的环金属化Pt(II)配合物的框架中,一系列双环金属化Pt(II)配合物[Pt(CNC)(L)]( L = DMSO,MeCN),其中包含各种(CNC)2–合成了基于2,6-二苯基吡啶的配体,并对其进行了分析和光谱表征,重点是它们的电化学,发光和抗增殖特性。电化学实验和紫外可见吸收光谱表明,与DFT计算一致,以配体为中心的LUMO和以金属为中心的HOMO。辅助苯基延伸至萘基核和中心的4-苯基吡啶基团而不是吡啶导致长波吸收带在420至440 nm范围内发生红移,后者的变化更为明显。稠合杂环数控系统DBA(H的配合物2 DBA =二苯并[ c ^,ħ ]吖啶),DB(PH)一(H 2分贝(PH)A = 7苯基二苯[ C ^,h ] ac啶)和bzqph(HbzqphH = 2-苯基苯并[ h ]喹啉)在500-530 nm范围内吸收更多的红移。所有的配合物都显示出可逆的首次电化学还原和不可逆的氧化,电化学间隙约为3 V,大致与吸收能一致。虽然2,6-二苯基吡啶配合物[Pt(CNC)(DMSO)]在环境温度下没有发光,但熔融的dba,db(ph)a和bzqph衍生物在环境温度下是有效的三重态发射体,发射波长为575-600 nm区域,量子产率为7%到23%。在DFT框架中计算得到的振动分辨发射光谱可以忠实地再现实验数据。激发态T 1下的TD-DFT计算几何形状揭示了所研究的所有三个复合物的发射的配体π–π * / MLCT特性。对选定的复合物进行的抗增殖测试产生了非常不同的毒性,范围从低于1μM到几乎无毒。尽管溶解度的变化也可以显着说明不同的毒性,但数据仍可得出一些构效关系(SAR)。
更新日期:2020-02-28
中文翻译:

CNC钳配体的环金属化Pt配合物:发光和细胞毒性评估
在我们尝试开发用于双功能靶向抑制剂或光动力疗法,诊断学和生物成像试剂的环金属化Pt(II)配合物的框架中,一系列双环金属化Pt(II)配合物[Pt(CNC)(L)]( L = DMSO,MeCN),其中包含各种(CNC)2–合成了基于2,6-二苯基吡啶的配体,并对其进行了分析和光谱表征,重点是它们的电化学,发光和抗增殖特性。电化学实验和紫外可见吸收光谱表明,与DFT计算一致,以配体为中心的LUMO和以金属为中心的HOMO。辅助苯基延伸至萘基核和中心的4-苯基吡啶基团而不是吡啶导致长波吸收带在420至440 nm范围内发生红移,后者的变化更为明显。稠合杂环数控系统DBA(H的配合物2 DBA =二苯并[ c ^,ħ ]吖啶),DB(PH)一(H 2分贝(PH)A = 7苯基二苯[ C ^,h ] ac啶)和bzqph(HbzqphH = 2-苯基苯并[ h ]喹啉)在500-530 nm范围内吸收更多的红移。所有的配合物都显示出可逆的首次电化学还原和不可逆的氧化,电化学间隙约为3 V,大致与吸收能一致。虽然2,6-二苯基吡啶配合物[Pt(CNC)(DMSO)]在环境温度下没有发光,但熔融的dba,db(ph)a和bzqph衍生物在环境温度下是有效的三重态发射体,发射波长为575-600 nm区域,量子产率为7%到23%。在DFT框架中计算得到的振动分辨发射光谱可以忠实地再现实验数据。激发态T 1下的TD-DFT计算几何形状揭示了所研究的所有三个复合物的发射的配体π–π * / MLCT特性。对选定的复合物进行的抗增殖测试产生了非常不同的毒性,范围从低于1μM到几乎无毒。尽管溶解度的变化也可以显着说明不同的毒性,但数据仍可得出一些构效关系(SAR)。

































 京公网安备 11010802027423号
京公网安备 11010802027423号