当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Condensation of 2-((alkylthio)(aryl)methylene)malononitrile with 1,2-aminothiol as a novel bioorthogonal reaction for site-specific protein modification and peptide cyclization
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-02-28 , DOI: 10.1021/jacs.9b11875
Xiaoli Zheng 1 , Zhuoru Li 1 , Wei Gao 1 , Xiaoting Meng 1, 2 , Xuefei Li 2 , Louis Y P Luk 2 , Yibing Zhao 1 , Yu-Hsuan Tsai 2 , Chuanliu Wu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-02-28 , DOI: 10.1021/jacs.9b11875
Xiaoli Zheng 1 , Zhuoru Li 1 , Wei Gao 1 , Xiaoting Meng 1, 2 , Xuefei Li 2 , Louis Y P Luk 2 , Yibing Zhao 1 , Yu-Hsuan Tsai 2 , Chuanliu Wu 1
Affiliation
|
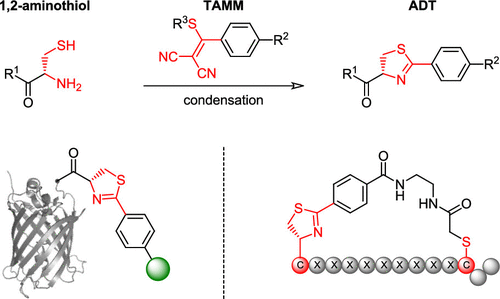
Site-specific modification of peptides and proteins has wide applications in probing and perturbing biological systems. Herein we report that 1,2-aminothiol can react rapidly, specifically and efficiently with 2-((alkylthio)(aryl)methylene)malononitrile (TAMM) under biocompatible conditions. This reaction undergoes a unique mechanism involving thiol-vinyl sulfide exchange, cyclization and elimination of dicyanomethanide to form 2-aryl-4,5-dihydrothiazole (ADT) as a stable product. An 1,2-aminothiol functionality can be introduced into a peptide or a protein as an N-terminal cysteine or an unnatural amino acid. The bioorthogonality of this reaction was demonstrated by site-specific labeling of not only synthetic peptides and a purified recombinant protein but also proteins on mammalian cells and phages. Unlike other reagents in bioorthogonal reactions, the chemical and physical properties of TAMM can be easily tuned. TAMM can also be applied to generate phage-based cyclic peptide libraries without reducing phage infectivity. Using this approach, we identified ADT-cyclic peptides with high affinity to different protein targets, providing valuable tools for biological studies and potential therapeutics. Furthermore, the mild reaction condition of TAMM condensation warrants its use with other bioorthogonal reactions to simultaneously achieve multiple site-specific modifications.
中文翻译:

2-((烷硫基)(芳基)亚甲基)丙二腈与 1,2-氨基硫醇的缩合作为位点特异性蛋白质修饰和肽环化的新型生物正交反应
肽和蛋白质的位点特异性修饰在探测和扰动生物系统中具有广泛的应用。在此我们报告了 1,2-氨基硫醇可以在生物相容性条件下与 2-((烷硫基)(芳基)亚甲基)丙二腈 (TAMM) 快速、特异性和有效地反应。该反应经历了一种独特的机制,包括硫醇-乙烯基硫化物交换、环化和二氰基甲烷的消除,以形成 2-芳基-4,5-二氢噻唑 (ADT) 作为稳定的产物。可以将 1,2-氨基硫醇官能团作为 N 端半胱氨酸或非天然氨基酸引入肽或蛋白质中。该反应的生物正交性通过不仅合成肽和纯化的重组蛋白而且哺乳动物细胞和噬菌体上的蛋白质的位点特异性标记来证明。与生物正交反应中的其他试剂不同,TAMM 的化学和物理特性可以轻松调整。TAMM 还可用于生成基于噬菌体的环肽文库,而不会降低噬菌体的感染性。使用这种方法,我们鉴定了对不同蛋白质靶标具有高亲和力的 ADT 环肽,为生物学研究和潜在治疗提供了有价值的工具。此外,TAMM 缩合的温和反应条件保证了其与其他生物正交反应的使用,以同时实现多个位点特定的修饰。我们鉴定了对不同蛋白质靶标具有高亲和力的 ADT 环肽,为生物学研究和潜在治疗提供了宝贵的工具。此外,TAMM 缩合的温和反应条件保证其与其他生物正交反应一起使用,以同时实现多个位点特异性修饰。我们鉴定了对不同蛋白质靶标具有高亲和力的 ADT 环肽,为生物学研究和潜在治疗提供了宝贵的工具。此外,TAMM 缩合的温和反应条件保证了其与其他生物正交反应的使用,以同时实现多个位点特定的修饰。
更新日期:2020-02-28
中文翻译:

2-((烷硫基)(芳基)亚甲基)丙二腈与 1,2-氨基硫醇的缩合作为位点特异性蛋白质修饰和肽环化的新型生物正交反应
肽和蛋白质的位点特异性修饰在探测和扰动生物系统中具有广泛的应用。在此我们报告了 1,2-氨基硫醇可以在生物相容性条件下与 2-((烷硫基)(芳基)亚甲基)丙二腈 (TAMM) 快速、特异性和有效地反应。该反应经历了一种独特的机制,包括硫醇-乙烯基硫化物交换、环化和二氰基甲烷的消除,以形成 2-芳基-4,5-二氢噻唑 (ADT) 作为稳定的产物。可以将 1,2-氨基硫醇官能团作为 N 端半胱氨酸或非天然氨基酸引入肽或蛋白质中。该反应的生物正交性通过不仅合成肽和纯化的重组蛋白而且哺乳动物细胞和噬菌体上的蛋白质的位点特异性标记来证明。与生物正交反应中的其他试剂不同,TAMM 的化学和物理特性可以轻松调整。TAMM 还可用于生成基于噬菌体的环肽文库,而不会降低噬菌体的感染性。使用这种方法,我们鉴定了对不同蛋白质靶标具有高亲和力的 ADT 环肽,为生物学研究和潜在治疗提供了有价值的工具。此外,TAMM 缩合的温和反应条件保证了其与其他生物正交反应的使用,以同时实现多个位点特定的修饰。我们鉴定了对不同蛋白质靶标具有高亲和力的 ADT 环肽,为生物学研究和潜在治疗提供了宝贵的工具。此外,TAMM 缩合的温和反应条件保证其与其他生物正交反应一起使用,以同时实现多个位点特异性修饰。我们鉴定了对不同蛋白质靶标具有高亲和力的 ADT 环肽,为生物学研究和潜在治疗提供了宝贵的工具。此外,TAMM 缩合的温和反应条件保证了其与其他生物正交反应的使用,以同时实现多个位点特定的修饰。































 京公网安备 11010802027423号
京公网安备 11010802027423号